无金属参与的以氧气为氧化剂的醇氧化体系研究毕业论文
2020-05-23 16:00:10
附件
一、产物表征
通过1H NMR光谱对所有的产物进行表征。
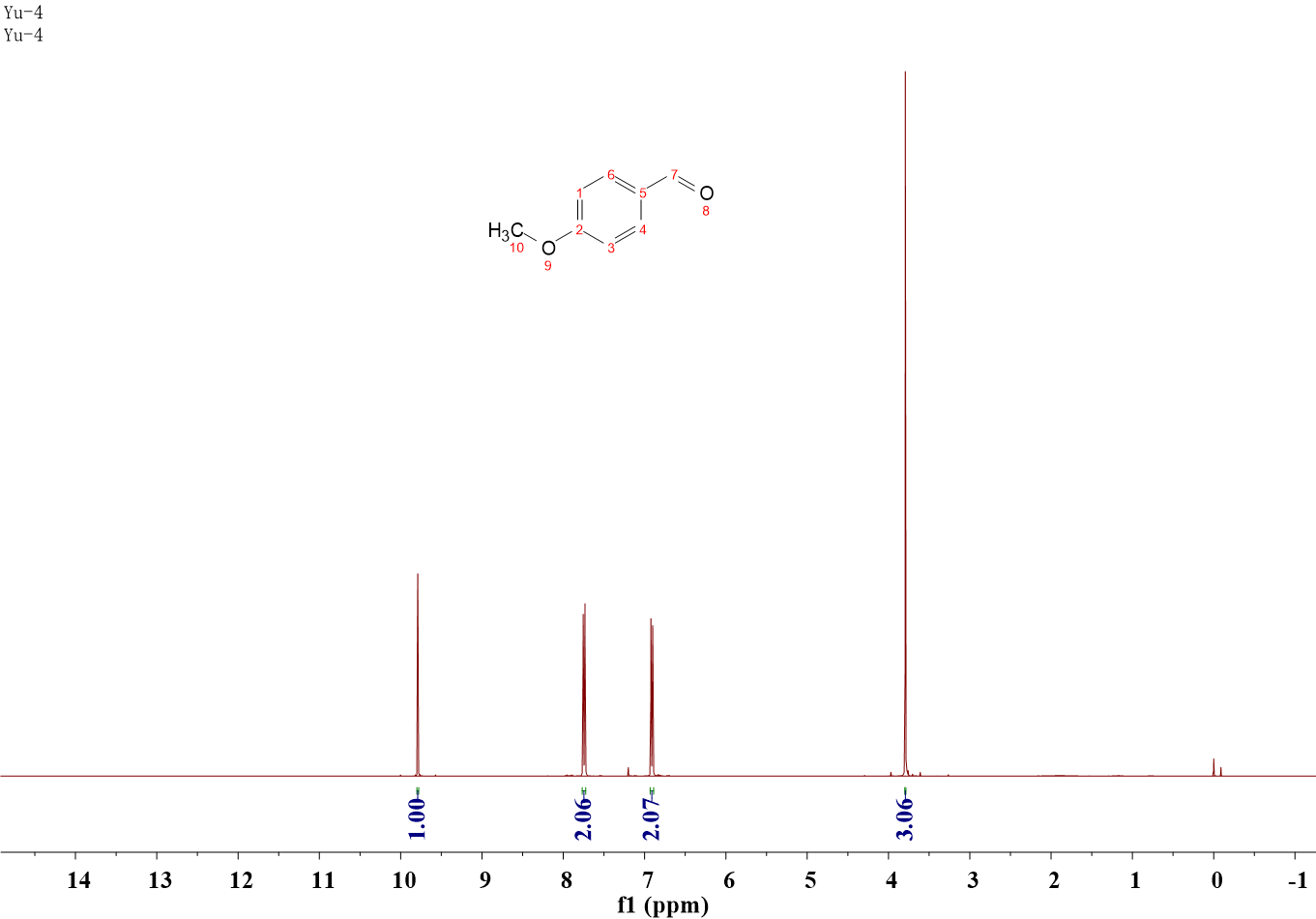
Anisic aldehyde
1H NMR (400 MHz, CDCl3) δ 9.79 (s, 1H), 7.77 – 7.72 (m, 2H), 6.94 – 6.89 (m, 2H), 3.79 (s, 3H).

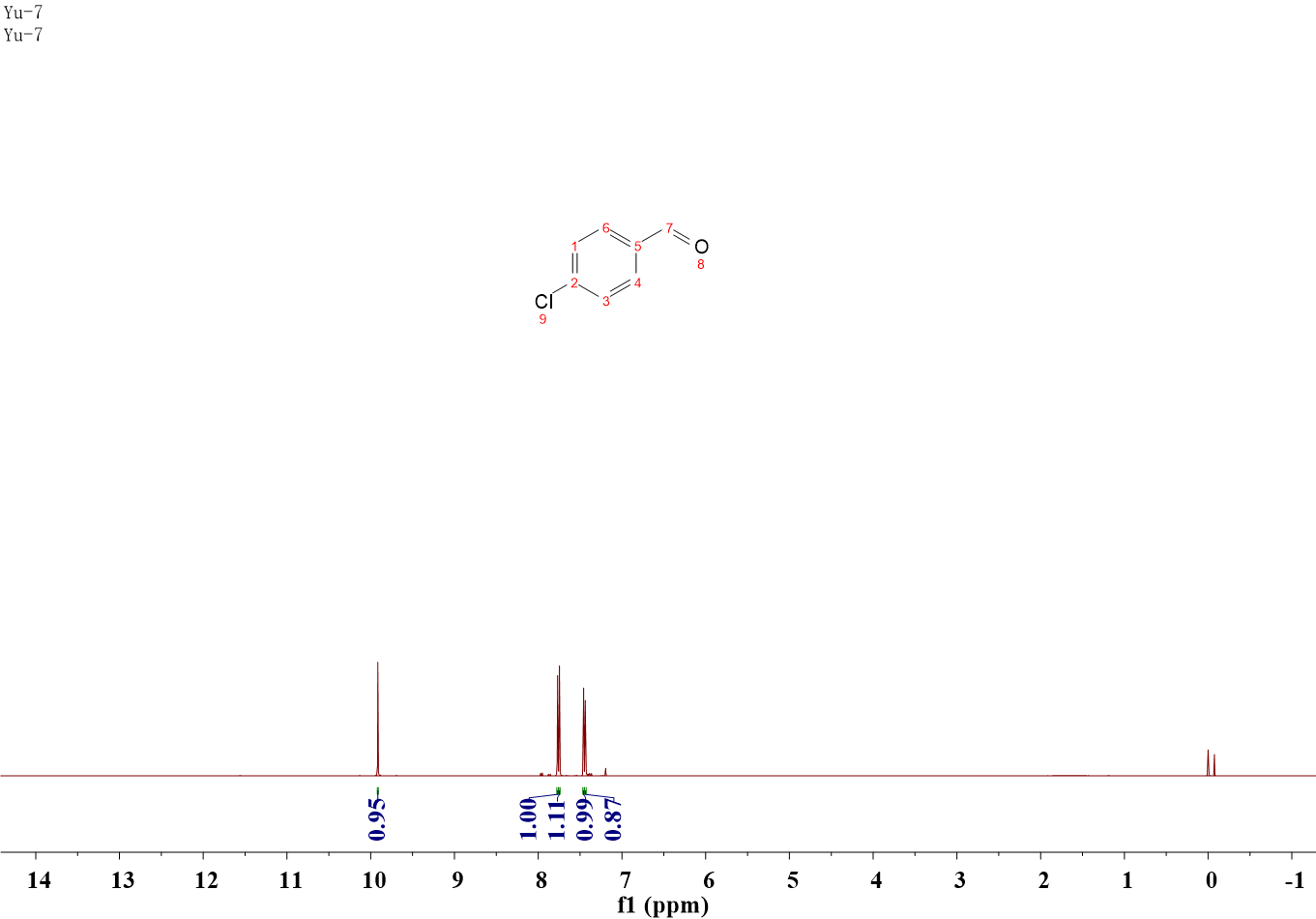
4-Chlorobenzaldehyde
1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 7.78 – 7.76 (m, 1H), 7.75 – 7.74 (m, 1H), 7.47 – 7.45 (m, 1H), 7.45 – 7.43 (m, 1H).

4'-Chloroacetophenone

1H NMR (400 MHz, CDCl3) δ 7.84 – 7.82 (m, 1H), 7.81 – 7.80 (m, 1H), 7.38 – 7.36 (m, 1H), 7.35 – 7.34 (m, 1H), 2.51 (s, 3H).
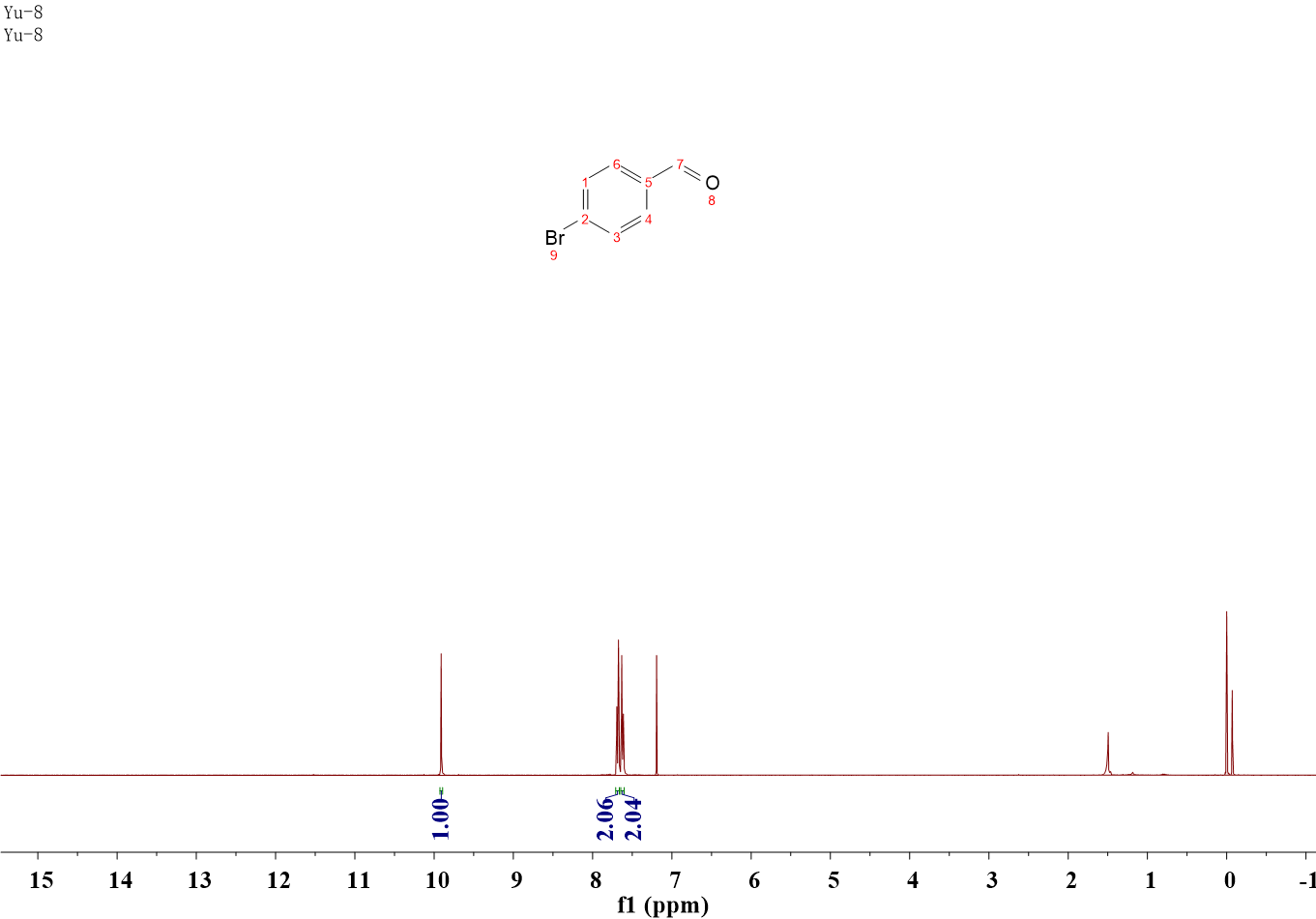
4-Bromobenzaldehyde
1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 7.68 (d, J = 8.3 Hz, 2H), 7.62 (d, J = 8.3 Hz, 2H).

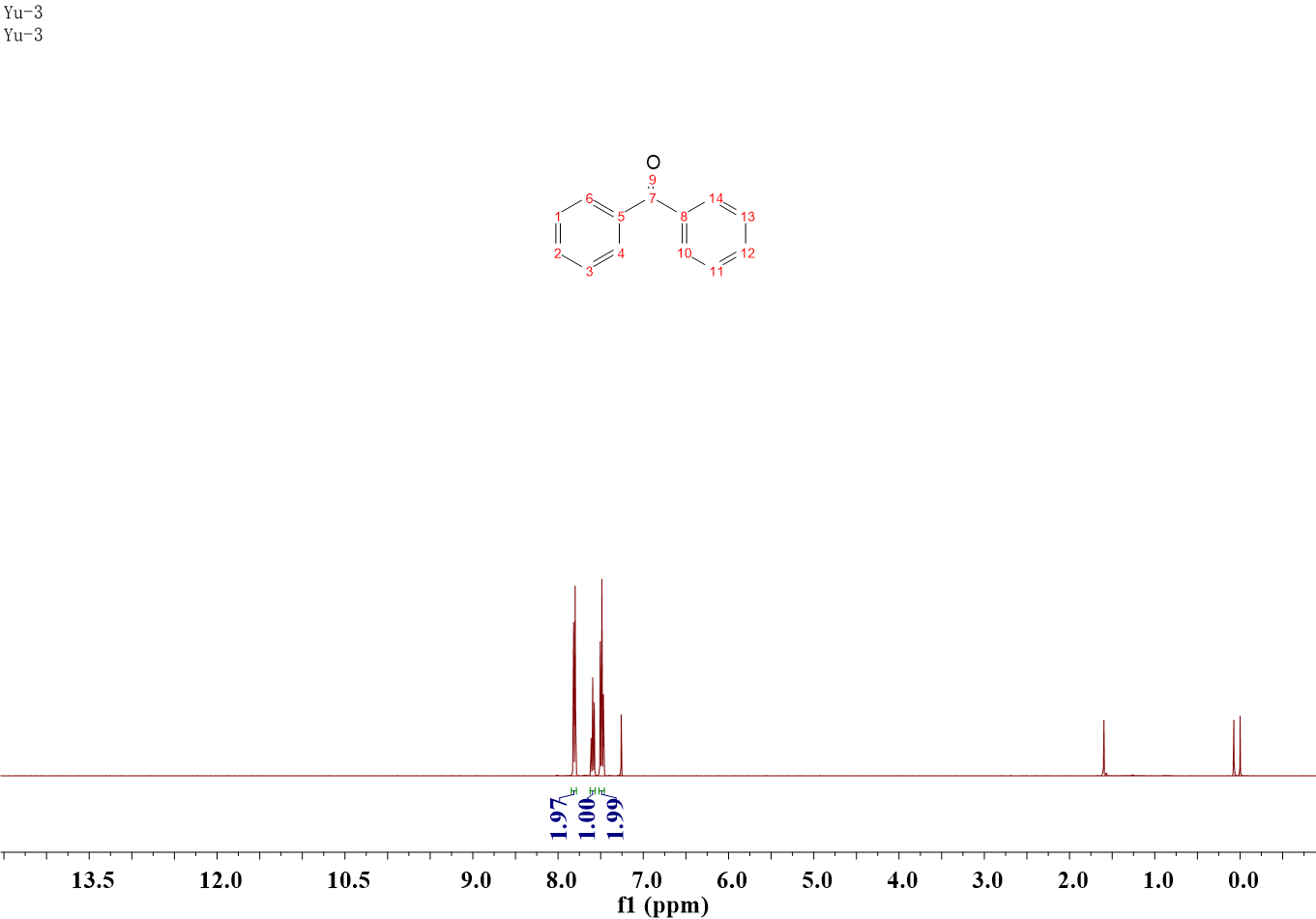
Benzophenone (2i)

1H NMR (400 MHz, CDCl3) δ 7.84 – 7.78 (m, 4H), 7.62 – 7.56 (m, 2H), 7.52 – 7.46 (m, 4H).
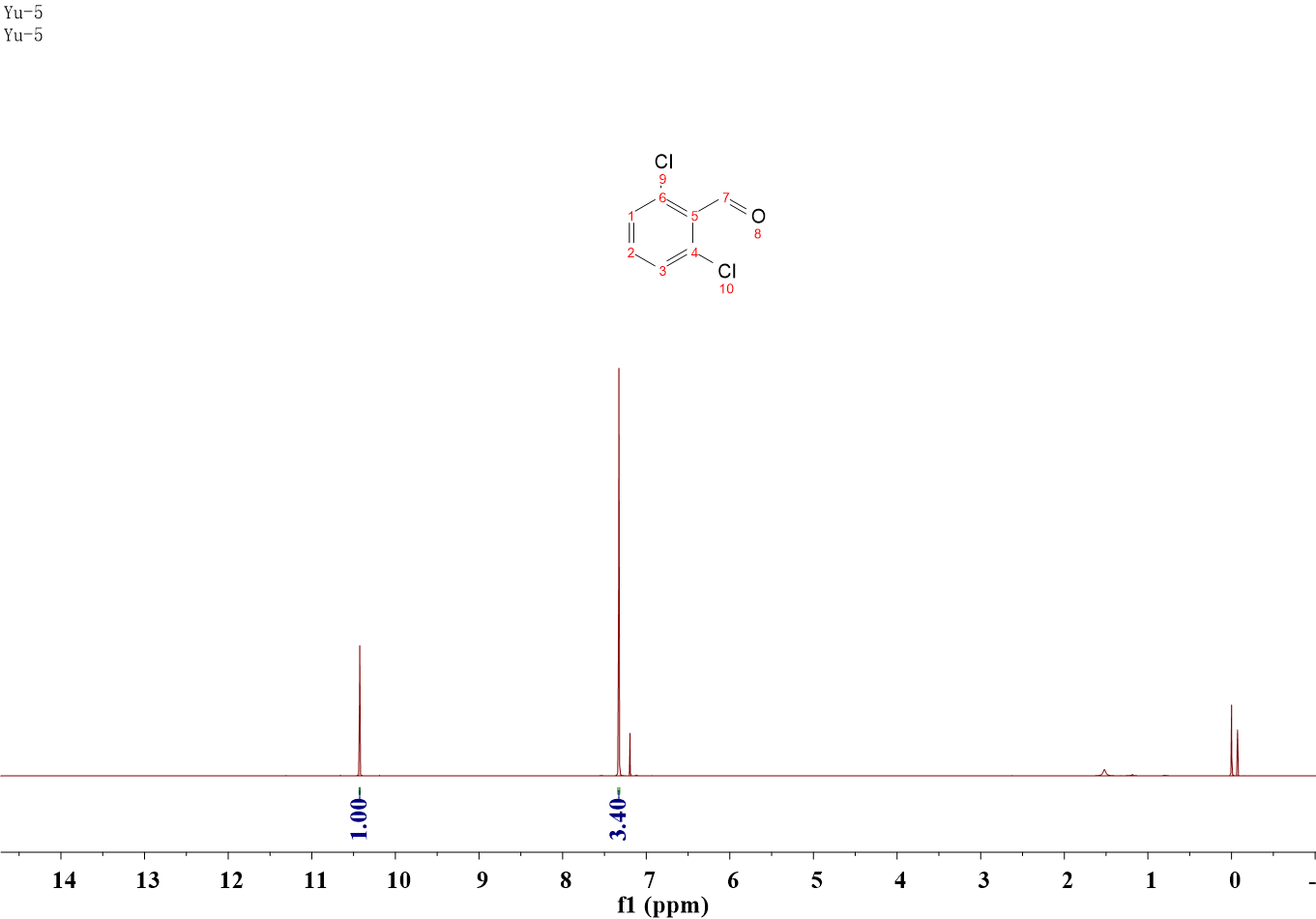
2, 6-Dichlorobenzaldehyde.
1H NMR (400 MHz, CDCl3) δ 10.43 (s, 1H), 7.33 (s, 3H).

Furfural
1H NMR (400 MHz, CDCl3) δ 9.59 (s, 1H), 7.64 – 7.62 (m, 1H), 7.19 (dd, J = 3.6, 0.5 Hz, 1H), 6.54 (dd, J = 3.6, 2 Hz, 1H).

2-Acetylthiophene
1H NMR (400 MHz, CDCl3) δ 7.63 (dd, J = 3.8, 1.1 Hz, 1H), 7.57 (dd, J = 5.0, 1.1 Hz, 1H), 7.06 (dd, J = 4.9, 3.8 Hz, 1H), 2.50 (s, 3H).

3-Acetylpyridine
1H NMR (400 MHz, CDCl3) δ 9.09 (d, J = 2.1 Hz, 1H), 8.71 (dd, J = 4.8, 1.6 Hz, 1H), 8.16 (dt, J = 8.0, 2.0 Hz, 1H), 7.36 (dd, J = 8.0, 4.8 Hz, 1H), 2.57 (s, 3H).

Benzaldehyde (2p)
1H NMR (400 MHz, CDCl3) δ 9.94 (s, 1H), 7.83 – 7.78 (m, 2H), 7.58 – 7.53 (m, 1H), 7.45 (t, J = 7.6 Hz, 2H).

2-Chlorobenzaldehyde

1H NMR (400 MHz, CDCl3) δ 10.41 (s, 1H), 7.84 (dd, J = 7.7, 1.7 Hz, 1H), 7.48 – 7.43 (m, 1H), 7.37 (dd, J = 8.0, 0.9 Hz, 1H), 7.31 (t, J = 7.5 Hz, 1H).
m-Tolualdehyde

1H NMR (400 MHz, CDCl3) δ 9.89 (s, 1H), 7.58 (m, 2H), 7.37 – 7.30 (m, 2H), 2.33 (s, 3H).
3-Chlorobenzaldehyde
1H NMR (400 MHz, CDCl3) δ 9.90 (s, 1H), 7.77 (t, J = 1.6 Hz, 1H), 7.69 (dt, J = 7.6, 1.3 Hz, 1H), 7.52 (ddd, J = 8.0, 2.4, 1.2 Hz, 1H), 7.41 (t, J = 7.8 Hz, 1H).

p-Tolualdehyde
1H NMR (400 MHz, CDCl3) δ 9.88 (s, 1H), 7.69 (d, J = 8.1 Hz, 2H), 7.25 (d, J = 7.9 Hz, 2H), 2.36 (s, 3H).

Acetophenone(2ab)
1H NMR (400 MHz, CDCl3) δ 7.88 (dt, J = 8.5, 1.7 Hz, 2H), 7.51 – 7.45 (m, 1H), 7.41 – 7.35 (m, 2H), 2.52 (s, 3H).

4-Fluoroacetophenone

1H NMR (400 MHz, CDCl3) δ 7.93 – 7.87 (m, 2H), 7.04 (m, 2H), 2.51 (s, 3H).
2-Acetylfuran
1H NMR (400 MHz, CDCl3) δ 7.53 – 7.51 (m, 1H), 7.12 (dd, J = 3.5, 0.8 Hz, 1H), 6.47 (dd, J = 3.5, 1.7 Hz, 1H), 2.41 (s, 3H).

2-Thenaldehyde
1H NMR (400 MHz, CDCl3) δ 9.87 (d, J = 1.2 Hz, 1H), 7.73 – 7.68 (m, 2H), 7.14 (dd, J = 4.8, 3.8 Hz, 1H).

3-Pyridinecarboxaldehyde

1H NMR (400 MHz, CDCl3) δ 10.06 (s, 1H), 9.02 (dd, J = 1.6 Hz,0.4 Hz,1H), 8.78 (dd, J = 4.8, 1.6 Hz, 1H), 8.11 (dt, J = 7.9, 2.0 Hz, 1H), 7.43 (dd, J = 7.9, 4.8 Hz, 1H).
1-Tetralone
1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 7.6 Hz, 1H), 7.38 (td, J = 7.5, 1.3 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 7.17 (t, J = 6.4 Hz, 1H), 2.88 (t, J = 6.1 Hz, 2H), 2.61 – 2.53 (t, J = 6 Hz, 2H), 2.10 – 2.01 (m, 2H).

1-Acetyl-4-formylbenzene

1H NMR (400 MHz, CDCl3) δ 10.04 (s, 1H), 8.04 (d, J = 8.3 Hz, 2H), 7.93 – 7.90 (m, 2H), 2.60 (s, 3H).
4-Bromobenzoic acid

1H NMR (400 MHz, CDCl3) δ 7.91 – 7.87 (m, 2H), 7.57 – 7.53 (m, 2H).
Benzoic acid

1H NMR (400 MHz, CDCl3) δ 8.06 (dd, J = 8.2, 1.1 Hz, 2H), 7.58 – 7.52 (m, 1H), 7.41 (t, J = 7.7 Hz, 2H).
2-Furoic acid
1H NMR (400 MHz, CDCl3) δ 9.81 (s, 1H), 7.59 – 7.57 (m, 1H), 7.28 – 7.26 (m, 1H), 6.50 (dd, J = 3.5, 1.7 Hz, 1H).

4-Chlorobenzoic acid

1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H).
p-Toluic acid
1H NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.1 Hz, 2H), 7.20 (d, J = 8.2 Hz, 2H), 2.36 (s, 3H).

p-Nitrobenzoic acid

1H NMR (400 MHz, CDCl3) δ 8.26 (m 2H), 8.23 – 8.19 (m, 2H).
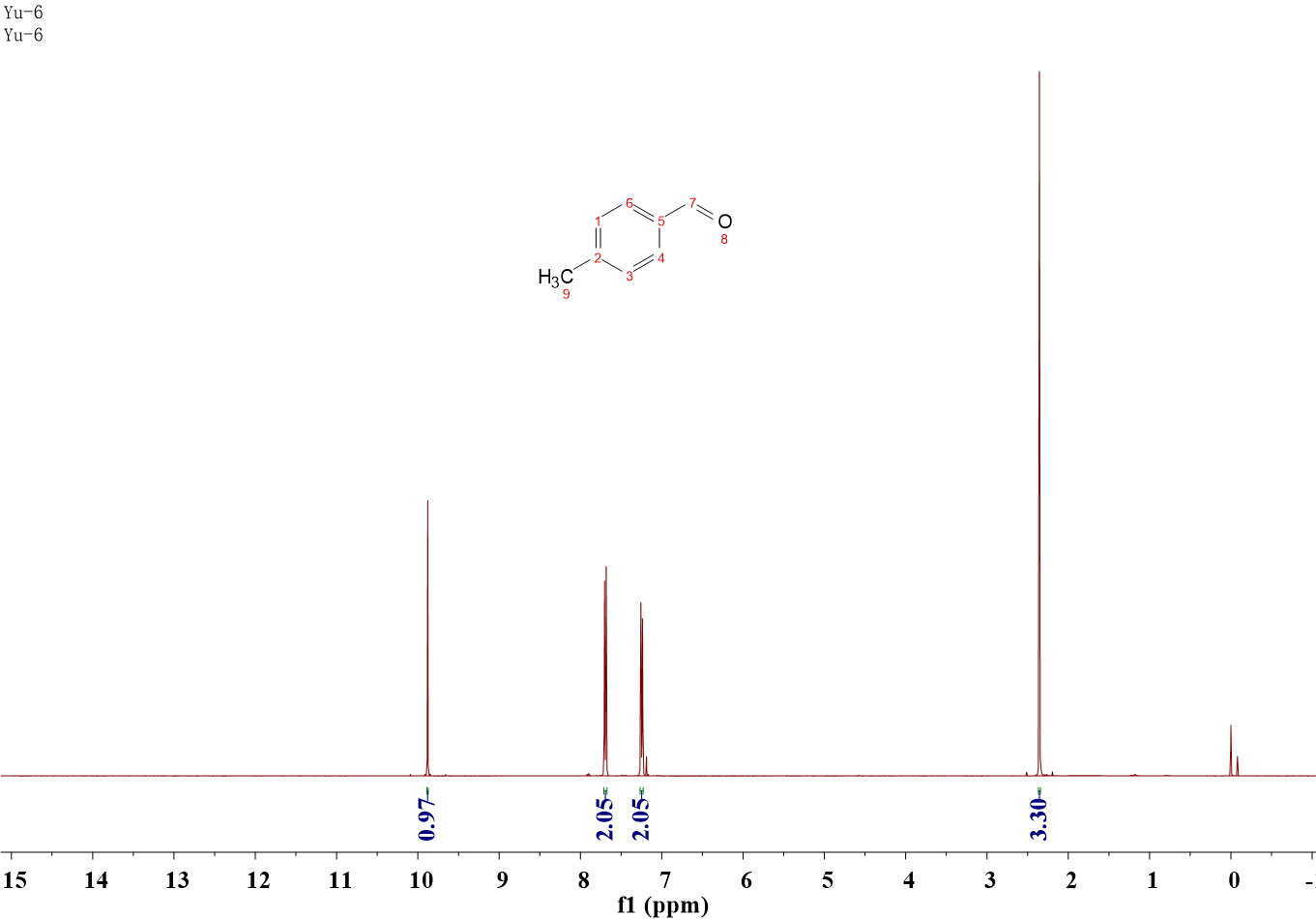
二、氢谱图
相关图片展示: