TiO2/Fe2o3复合材料的制备及其性能研究毕业论文
2021-12-09 17:33:18
论文总字数:30159字
摘 要
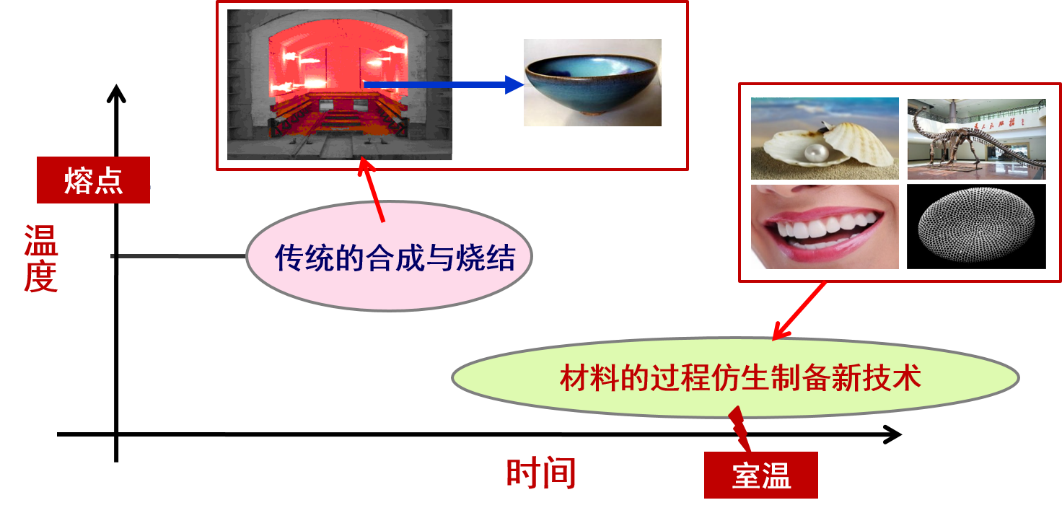
自然物质精妙的结构形成过程值得学习,以发展材料的制备新技术,我们称其为“材料的过程仿生制备技术”,意在发展颠覆性的室温或低温合成与加工技术。生物矿物是一种典型的有机-无机复合材料,具有完美的结构和优异的力学性能,而恰恰是这些少量的有机基质,在生物矿化过程中起着至关重要的作用。本研究工作中,以仿生物过程的合成为理念,学习生物矿物形成的重要特征:有机质在生物矿物的形成中起着至关重要的作用。选取天然绿叶及功能重组蛋白分别作为有机调控剂,指导TiO2/Fe2O3复合材料的合成,低温下制备出结构新颖的TiO2/Fe2O3复合材料。
首先,选取廉价的氯化铁、钛酸四丁酯作为主要原料,采用传统的水热合成制备TiO2/Fe2O3纳米复合材料。研究原料配比、温度及保温时间对产物结构和性能的影响,为后续研究提供了实验数据支撑。
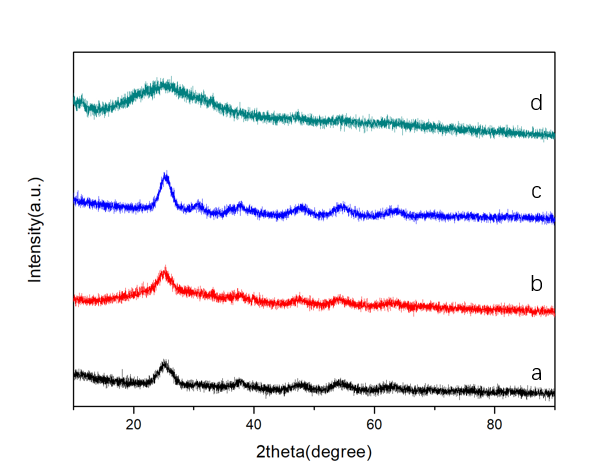
此外,首次采用植物的绿叶作为生物模板,指导TiO2/Fe2O3复合材料的合成。研究表明不同浓度的绿叶汁对产物的微观结构和晶型具有调控作用。随着绿叶汁浓度的增加,所得样品的结晶程度逐渐提高。当V银杏叶汁:V钛酸四丁酯=20:1时,得到分散性好的纯相TiO2颗粒。遗憾的是,未能得到预期的TiO2/Fe2O3纳米复合结构,所得粉体光催化活性较差。
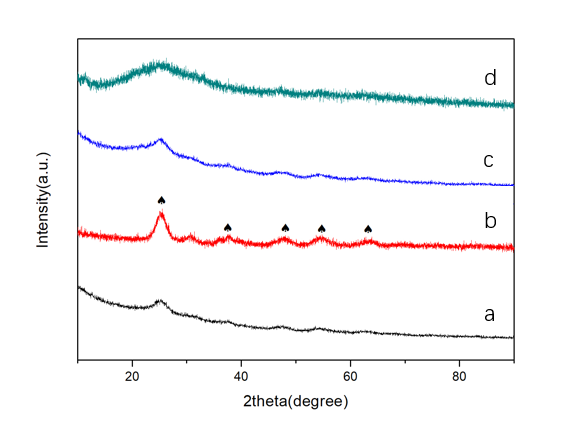
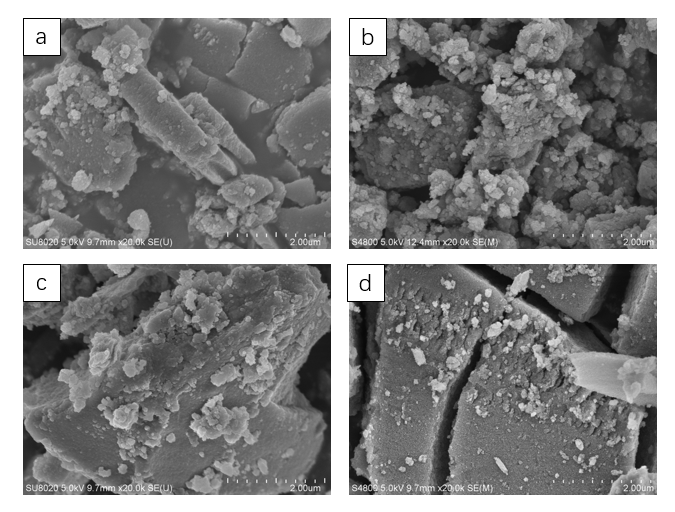
最后,基于指导贝壳形成的天然蛋白,成功设计制备了多功能重组蛋白CCaS(几丁质-钙片段-丝蛋白片段)。首次采用该多功能蛋白指导TiO2/Fe2O3纳米复合材料的合成,低温下成功制备出TiO2/Fe2O3复合粉体,且单一合成过程对TiO2/Fe2O3的晶型、微观结构及光催化性能同时起到了调控作用。研究表明:随着保温时间的延长,TiO2由无定形向锐钛矿,最后向金红石转变。当合成温度为120℃、保温时间为12 h时,得到了具有新颖独特结构的TiO2/Fe2O3的复合粉体:无定形TiO2纳米颗粒均匀覆盖在Fe2O3叠层纳米棒上。该粉体具有优异的光催化性能(45 min可降解94%罗丹明B)。
本研究工作所得的结果扩展了目前材料制备的方法,特别是为低温或室温下设计和制备复合材料粉体带来了无数的可能性。
关键词:TiO2/Fe2O3复合材料;光催化;水热反应;绿叶汁;CCaS重组蛋白
Abstract
The exquisite structure formation process of natural materials is worth studying to develop new technologies for the preparation of materials. We call it "the bionic preparation technology of materials." "Bio-process bionic preparation technology" is the first new idea and new research direction proposed by Chinese scientists in the world. It will develop subversive room temperature or low temperature synthesis and processing technology. Biomineral is a typical organic-inorganic composite material with perfect structure and excellent mechanical properties. It is precisely these small organic matrices that play a vital role in the biomineralization process. In this research work, the concept of biomimetic process synthesis is used to learn the important characteristics of biomineral formation: organic matter plays a vital role in the formation of biominerals. Natural green leaves and functional recombinant proteins were selected as organic regulators to guide the synthesis of TiO2/Fe2O3 composites, and novel structures of TiO2/Fe2O3 composites were prepared at low temperatures.
Firstly, the cheap ferric chloride and tetrabutyl titanate were selected as the main raw materials, and traditional hydrothermal synthesis was used to prepare TiO2/Fe2O3 nanocomposites. The effects of raw material ratio, temperature and holding time on the structure and properties of the product were studied, and the technological conditions for the formation of TiO2/Fe2O3 composite materials were explored to provide experimental data support for subsequent research.
In addition, for the first time, green leaves of plants were used as biological templates to guide the synthesis of TiO2/Fe2O3 nanocomposites. The effects of different concentrations of green leaf juice and synthesis temperature on the microstructure and crystal form of TiO2/Fe2O3 products were studied. Studies have shown that as the volume of leaf juice added increases, the degree of crystallinity of the obtained sample gradually increases. The experimental parameters are V ginkgo leaf juice: V tetrabutyl titanate = 20: 1. The best state is TiO2 particles with good dispersion of pure phase. Unfortunately, the expected TiO2/Fe2O3 nanocomposite structure was not obtained, and the resulting powder had poor photocatalytic activity.
Finally, based on the natural protein that guides shell formation, a multifunctional recombinant protein CCaS was successfully designed. Preliminary attempts have been made to use this multifunctional protein to guide the synthesis of TiO2/Fe2O3 nanocomposites. TiO2/Fe2O3 composite powders have been successfully prepared at low temperatures, and the crystal form, microstructure and photocatalytic performance of TiO2/Fe2O3 have been successfully prepared in a single synthesis process. To many aspects of regulation and control. The results show that with the extension of the holding time, the TiO2 changes from amorphous to anatase, then from anatase to rutile, and finally all becomes rutile. Hematite transforms from laminated nanorods to single rods and finally to flakes. The TiO2/Fe2O3 powder prepared when the synthesis temperature is 120 ° C and the synthesis time is 12 h has a novel and unique composite structure: amorphous TiO2 Nanoparticles are uniformly coated on the Fe2O3 laminated nanorods, and have excellent photocatalytic performance. Within 45 minutes, a 0.02 g sample can degrade 94 ml of rhodamine B at a concentration of 0.01 g / ml.
The results of this research work extend the current methods of material preparation, and in particular, bring countless possibilities for designing and preparing composite powders under environmentally friendly conditions.
Key words: TiO2/Fe2O3 composite; photocatalysis; hydrothermal reaction; green leaf juice; CCaS recombinant protein
目录
第1章 绪论 1
1.1 背景 1
1.2 TiO2材料的简介 1
1.3 TiO2的改性 2
1.3.1 TiO2掺杂改性的作用原理 3
1.3.2 掺杂物的种类 3
1.3.3 负载 3
1.3.4 光敏化 4
1.4 TiO2/Fe2O3纳米复合材料 4
1.4.1 Fe2O3光催化剂 4
1.4.2 TiO2/Fe2O3纳米复合材料 4
1.4.3 TiO2/Fe2O3纳米复合材料的制备方法 5
1.5 材料的过程仿生制备 6
1.5.1 有机模板调控合成TiO2/Fe2O3纳米复合材料 7
1.6 研究目的、意义和内容 8
1.6.1 研究目的和意义 8
1.6.2 主要研究内容 8
第2章 实验方案设计 10
2.1 实验试剂及仪器 10
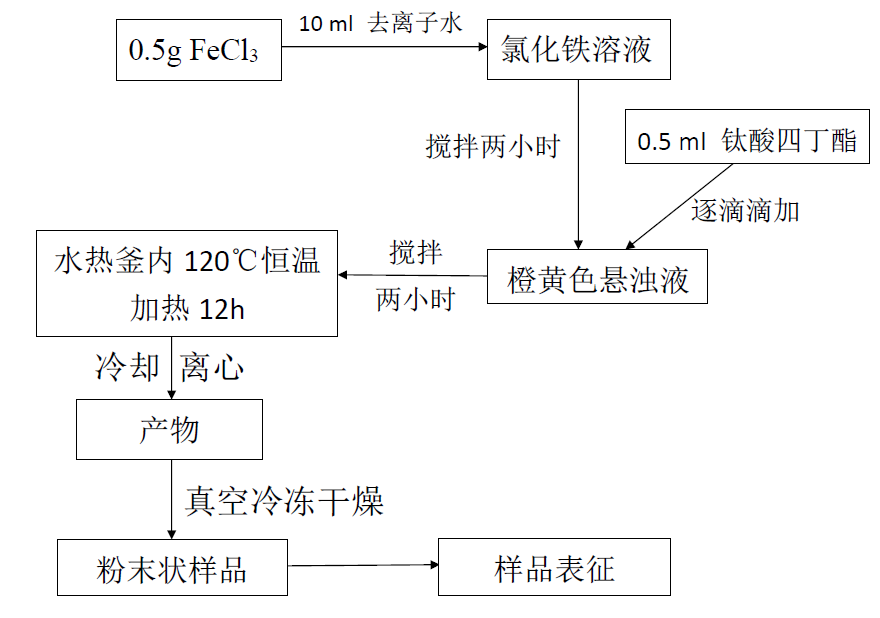
2.2 实验方案 10
2.3 表征测试方法 11
2.3.1 X射线衍射分析(XRD) 11
2.3.2 场发射扫描电镜(FESEM) 12
2.3.3 紫外-可见分光光度计 12
第3章 TiO2/Fe2O3复合材料的合成与结构调控研究 13
3.1 引言 13
3.2 实验方案 13
3.3 结果与讨论 14
3.4小结 20
第4章 有机质调控TiO2/Fe2O3复合材料的合成 21
请支付后下载全文,论文总字数:30159字
相关图片展示: