MoS2空心球的合成及其电催化性能的研究毕业论文
2020-06-16 20:51:45
摘 要
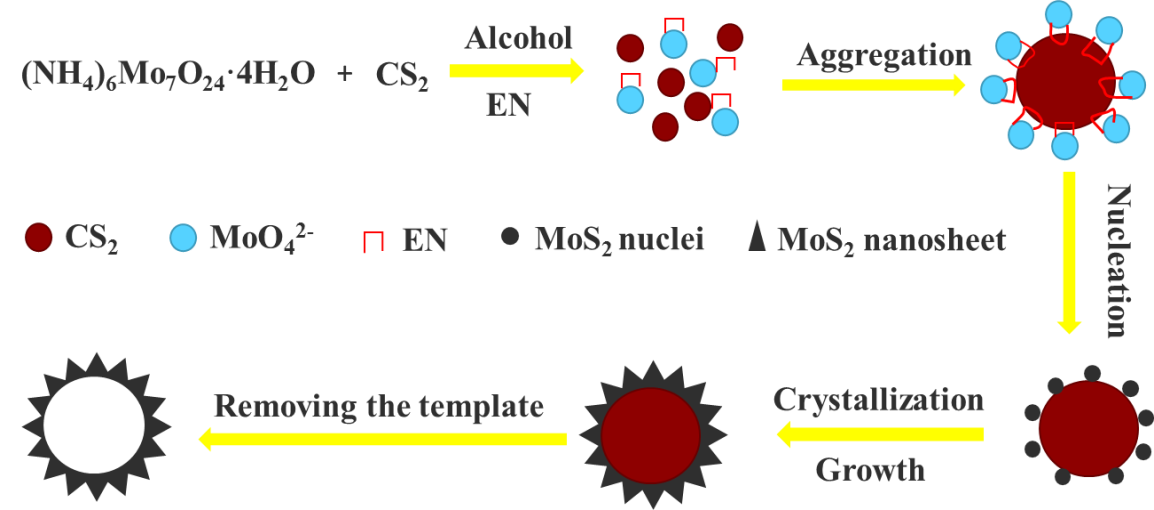
氢气是一种清洁燃料,由于其高热值和环境友好性,未来将被视为传统化石燃料的潜在的替代品。电催化析氢反应(HER)是生产氢的最重要的方法之一,可以有效地储存来自太阳能等可再生能源的能量,但受到Pt基贵金属催化剂的天然稀缺性和高成本的限制。因此,越来越多的科研工作者注意到非Pt阴极催化剂。近年来,基于MoS2的电催化剂由于其低成本以及与Pt相似的氢原子结合能,具有高的HER活性,引起了人们的关注。然而,MoS2纳米片易于团聚,导致活性位点的减少和催化性能的降低。因此,本文通过CS2辅助水热法成功制备了由二维MoS2纳米片构成的三维(3D)空心纳米球,“钼进入反应”机制可以得到了丰富的缺陷。由一系列少数几层的,缺陷丰富的MoS2纳米片构成的三维结构可以保持纳米片大量暴露的活性位点,并防止纳米片聚集,同时3D纳米结构有利于电解液渗透的介孔,故具有优异的催化性能。
关键词:析氢反应;MoS2;纳米空心球;催化剂
Synthesis of MoS2 Hollow Spheres and Study of Their Electrocatalytic Properties
Abstract
Hydrogen is a clean fuel and considered as a promising alternative for traditional fossil fuels in the future due to its high calorific value and environmental friendliness. Electrocatalytic hydrogen evolution reaction (HER) is one of the most important ways to produce hydrogen that may efficiently store energy from renewable sources such as solar energy, but limited by the natural scarcity and high cost of Pt-based noble metal catalyst. As a result, more and more scientific research workers have pay attention to non-Pt cathodic catalysts. In recent years, low-cost MoS2-based electro- catalysts have been attracting particular attention due to their similar hydrogen binding energy to that of Pt and hence high HER activity. However, the MoS2 nanosheets are easy to accumulate, resulting in the reduction of the active sites and the catalytic properties. Therefore, in this paper, the three-dimensional (3D) hollow nanopheres with two-dimensional MoS2 nanosheets were successfully prepared by a facile CS2-assisted hydrothermal method, and the “inverted molybdenum reaction” achieves a defect-rich structure. The three-dimensional architectures constructed from a series of few-layered and defect-rich MoS2 nanosheets, could maintain a large amount exposed active sites of nanosheets and prevent the nanosheets against agglomerating, in the meantime the 3D nanostructures have the advantage of suitable mesopores for electrolyte solution penetration, resulting in extraordinary catalytic performance.
Key words: hydrogen evolution reaction; MoS2; nanospheres; catalysts
目录
摘要 I
Abstract II
第一章:绪论 1
1.1选题背景及意义 1
1.2 MoS2的结构与性质 2
1.3 MoS2的制备方法 4
1.4 MoS2的应用、研究现状 4
第二章:MoS2空心球与片的制备 9
2.1.概述 9
2.2 实验部分 9
2.2.1 实验药品与仪器 9
2.2.2 实验步骤 9
2.3表征方法 10
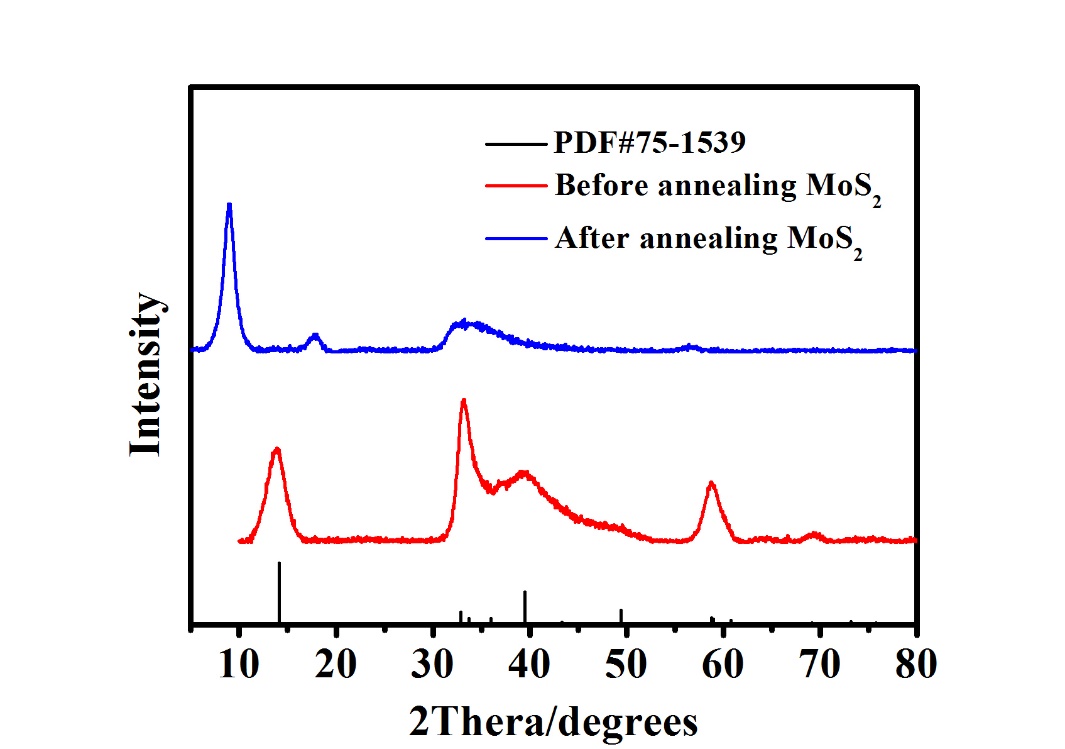
2.3.1 X射线衍射光谱(XRD) 10
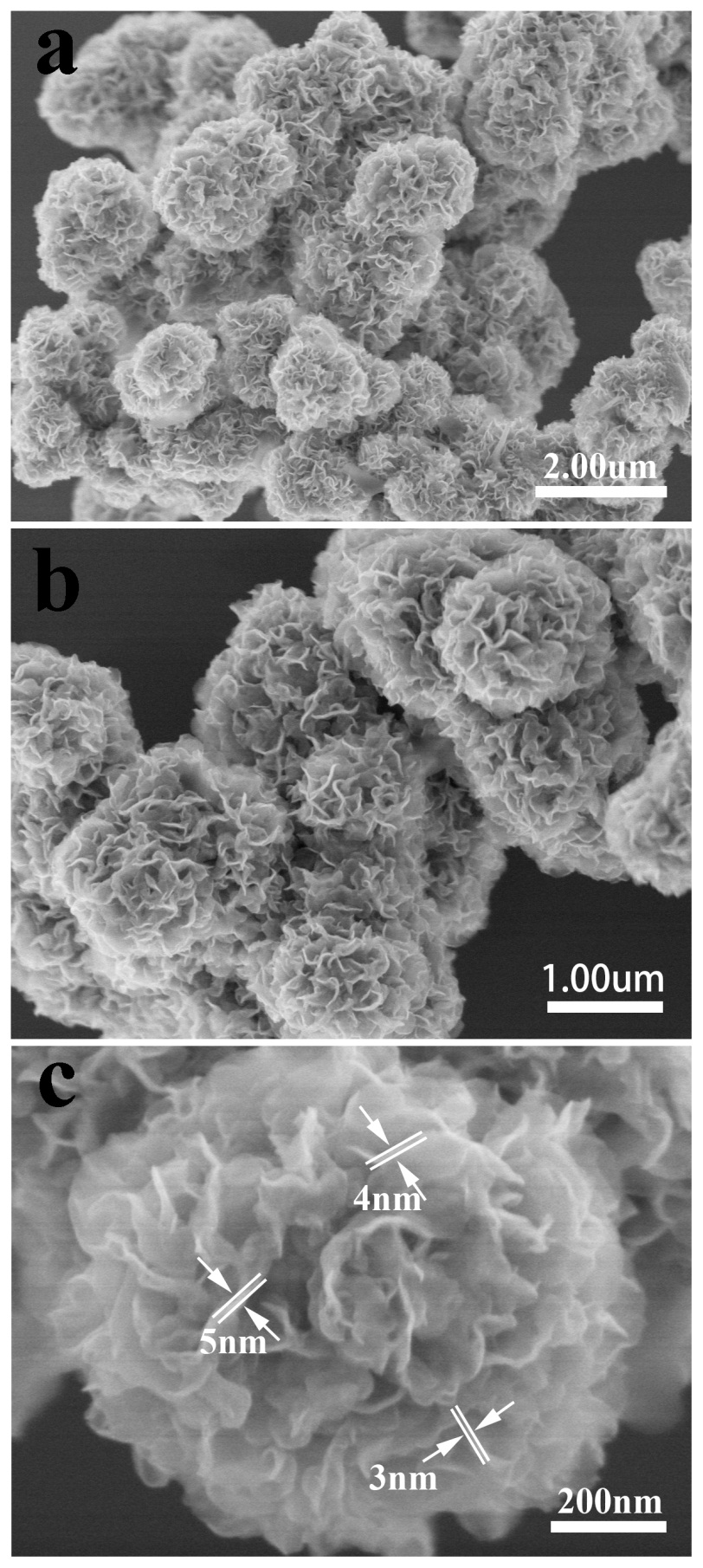
2.3.2场发射 11
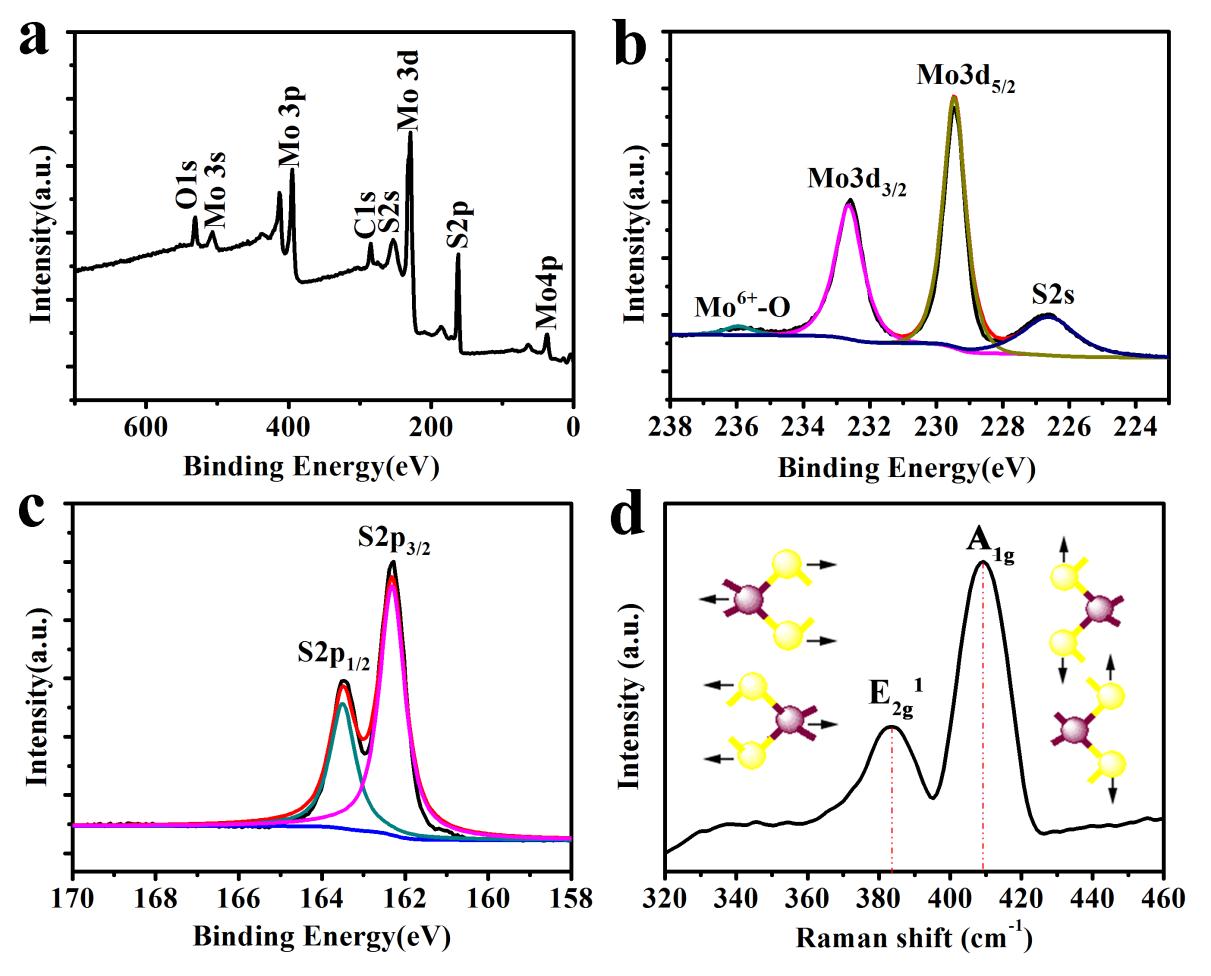
2.3.3 X射线光电子能谱分析(XPS) 11
2.3.4拉曼光谱 11
2.4 催化性能测试 11
2.4.1 电化学性能测试系统 11
2.4.2玻碳电极的打磨制作 11
2.4.3 线性扫描伏安法(LSV) 12
2.4.4 循环伏安法(CV) 12
2.4.5 交流阻抗法(EIS) 12
第三章:表征与性能测试 13
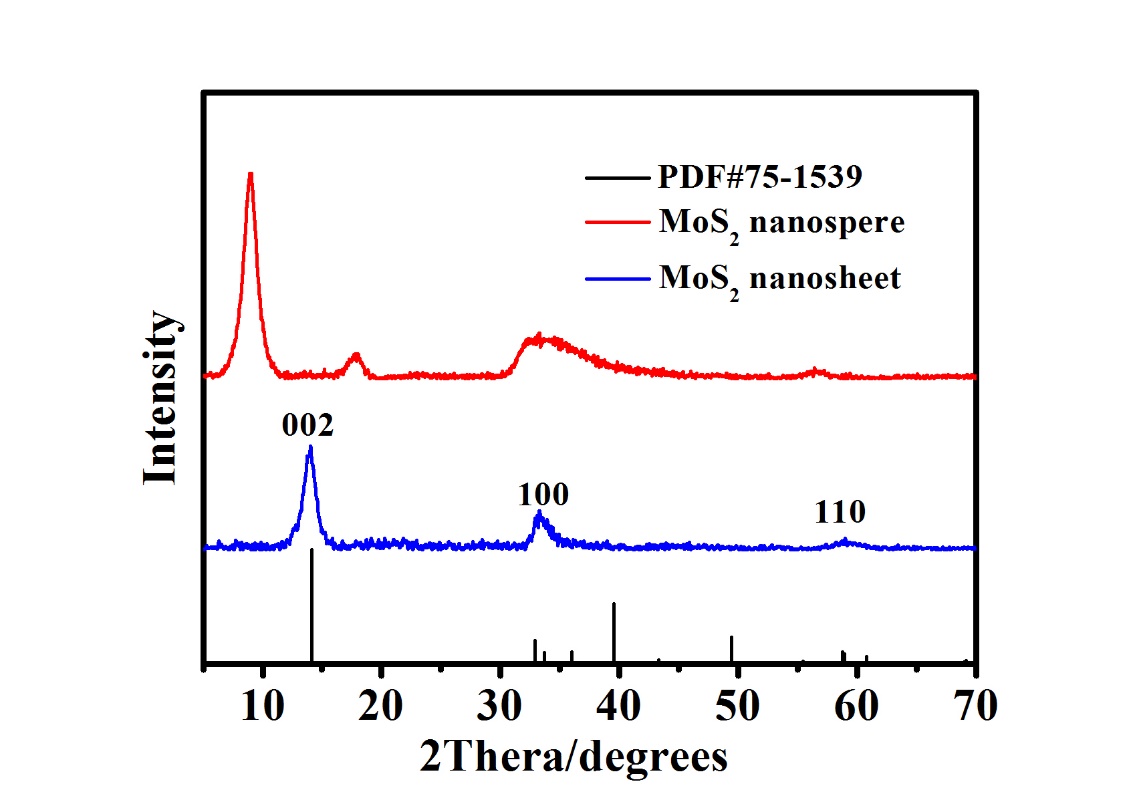
3.1 表征 13
3.1.1 XRD 13
3.1.2 场发射 14
3.1.3 XPS 15
3.1.4 拉曼光谱 16
3.2电催化性能测试 16
3.2.1 线性扫描伏安(LSV)曲线与Tafel斜率的测定 16
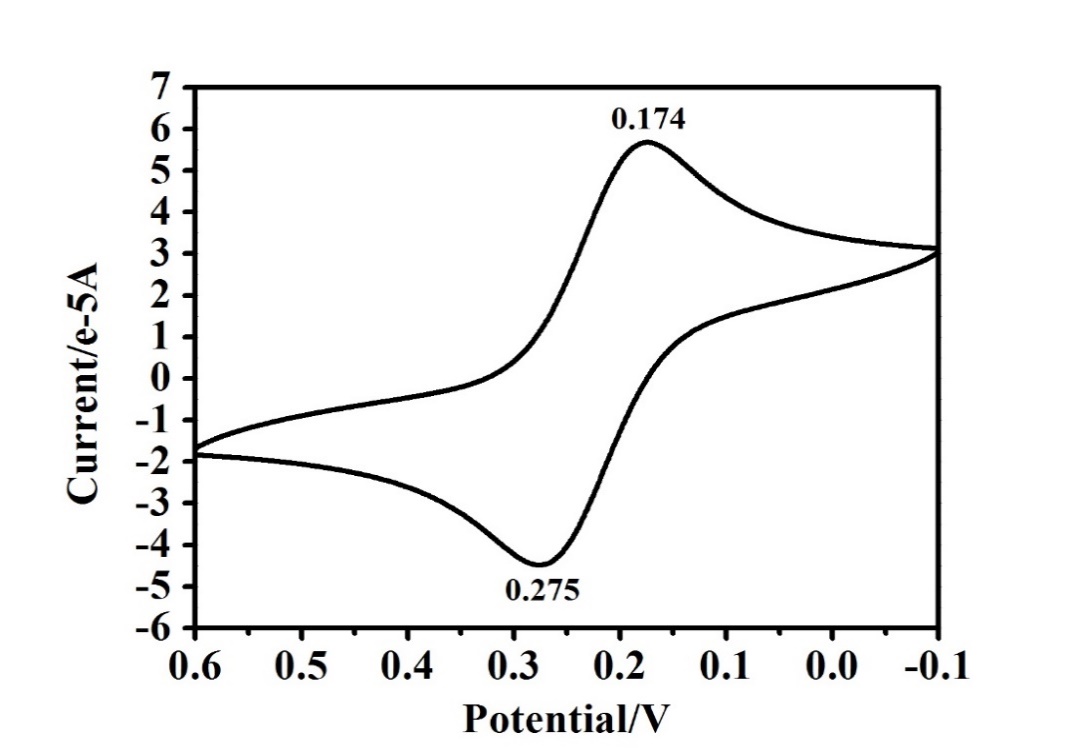
3.2.2 循环伏安(CV)曲线的测定 18
3.3.3 交流阻抗谱的测定 20
第四章:结果分析与讨论 21
4.1性能对比 21
4.2原因分析与总结 21
参考文献 22
致谢 25
第一章:绪论
1.1选题背景及意义
能源作为21世纪的支柱之一,其重要性可想而知。第一次工业革命之后,社会发展对于能源的需求越来越大,大量化石燃料的燃烧给环境带来了难以挽回的灾难,而且其作为不可再生,越用越少,故能源危机与环境危机正在危害人类的未来,寻找环保可循环能源显得尤为重要[1]。
氢气是一种有效,环保,可再生能源,并且作为燃料,燃烧效率高,储量丰富,燃烧后不产生环境污染 [2]。探寻高效节能的氢气制备方法已经成为当前的研究热点之一[3]。制氢的方法很多[4],比较常见的有:
(1)化石燃料制氢,主要有天然气蒸汽转化,重油部分氧化等[5]。
(2)生物质制氢,即分解各种生物质来制取氢气。
(3)多种化工过程制氢,如在氯碱工业中,通过提纯副产物制氢。其主要反应为:
在阳极上 2Cl− == Cl2 2e−
在阴极上 2H 2e− == H2
相关图片展示: