脲酶驱动双面神纳米金马达的制备及运动性能研究毕业论文
2021-12-10 17:44:24
论文总字数:39605字
摘 要
纳米马达是一种尺寸在纳米级、通过将环境中其它形式的能量转化为自身动能实现自主运动的纳米器件。由于其特殊的自主运动性能,在生物传感、主动给药及微创手术等生物医药领域具有良好的应用前景。然而,目前研究的马达大多集中于微米尺寸且构筑马达的材料及驱动马达的燃料具有细胞毒性,故而并不宜在人体内环境中应用。 鉴于此,本文提出在纳米金表面一半修饰高分子聚乙二醇(PEG),另一半偶联脲酶以形成不对称纳米结构,通过脲酶催化尿素分解作为驱动力的脲酶驱动纳米金马达,该马达动力强、生物相容性好,适合用于生物环境中。具体研究内容如下:
第一,通过种子生长法制备多种尺寸纳米金,并取其中平均粒径为27.5 nm及48 nm的纳米金分别作为构建马达的基本材料,运用静电吸附法及巯基与纳米金的键合作用,制得PEG@Au纳米不对称结构,再利用EDC/NHS法将脲酶偶联于其上得到脲酶驱动纳米金马达。通过测量Zeta电位及水合粒径验证了纳米不对称结构的成功建立,运用两种尺寸的纳米金均构建出了马达证明本制备方案的普适性。本方案中所选材料均具有生物相容性,适合运用于生物医药领域中。
第二,通过暗场显微镜及动态光散射技术对马达的运动进行表征,两种尺寸的马达在尿素溶液中均表现为增强扩散,其运动均随尿素浓度的升高而加快,当尿素达到一定浓度后马达速度基本保持不变,这一现象符合了米氏定律。27.5 nm纳米金制得的脲酶驱动纳米马达在20 mM尿素溶液中平均速度达到最大,为6.23 ± 2.22 μm/s,约207体长/s,其扩散相较于其布朗运动(0.485 ± 0.160 μm2/s)最大增强了109.28%,达1.015 ± 0.253 μm2/s;48 nm纳米金制得的脲酶驱动纳米马达在10 mM尿素溶液中平均速度达到最大,为5.92 ± 1.53 μm/s,约为118体长/s,其扩散相较于其布朗运动(0.456 ± 0.102 μm2/s)最大增强了94.96%,达0.889 ± 0.244 μm2/s。该马达的扩散增强程度高于目前研究的纳米马达,具有更强的驱动力,为日后在复杂的人体内环境中应用提供了可能。
关键词:纳米马达;纳米金;脲酶驱动;增强扩散;生物相容性
Abstract
Nanomotors are nanomachines at the nanometer scale, being able to convert other forms of energy into mechanical energy and thus realizing self-motion. Due to their unique self-motion performance, nanomotors may find applications in biosensor, drug delivery, microsurgery and other biomedical fields. However, most of the currently studied motors are at microscale and their materials and fuels are cytotoxicity. As a result, these kinds of motors are unsuitable for application in human internal environments. Herein, this paper presents a design of asymmetric nanostructure with gold nanoparticles. On half of the surface of gold nanoparticles is modified with polyethylene glycol (PEG), while urease is coupled on the other half of the surface. This urease-powered gold nanomotor is driven by catalytic reaction between urease and urea and have a strong diving force and good biocompatibility so that it is suitable for using in biological environments. The specific research contents are as followed:
Firstly, different sizes of gold nanoparticles are synthesized following seeded growth strategy and 27.5 nm as well as 48 nm gold nanoparticles are used as the material of nanomotor. Using electrostatic adsorption and bonding effects between sulfydryl and gold, the asymmetric nanostructure PEG@Au is fabricated. And then urease can be immobilized on the surface of gold particles via EDC/NHS strategy to successfully design an urease-powered gold nanomotors. The success of fabrication of asymmetric nanostructure is proved by measuring Zeta potentials and hydrated particle sizes of productions. The scheme in this paper is universal because two different sizes gold nanoparticles both successfully fabricate nanomotors. The materials used in this scheme are all biocompatible so that this nanomotor has potential applications in biomedical fields.
Secondly, the motion of nanomotors is detected by dark field microscope and dynamic light scattering. Both two size nanomotors exhibit enhanced diffusion in urea solution. The motion of them increases with the concentration of urea ascending and finally become constant. This phenomenon obeys the Michaelis-Menten equation. The average velocity of urease-powered nanomotors made by 27.5 nm gold nanoparticles reaches the maximum value, at 6.23 ± 2.22 μm/s, in 20 mM urea. The diffusion of it is enhanced by 109.28% to 1.015 ± 0.253 μm2/s, comparing to its Brownian diffusion constant(0.485 ± 0.160 μm2/s). By comparison, the average of urease-powered nanomotors made by 48 nm gold nanoparticles grow to maximum in 10 mM urea, about 5.92 ± 1.53 μm/s. And the diffusion of that is enhanced by 94.96% to 0.889 ± 0.244 μm2/s, comparing to its Brownian diffusion constant(0.485 ± 0.160 μm2/s). The nanomotors designed in this paper have an obviously higher enhanced diffusion and stronger driven force comparing with other nanomotors so that they provide possibility for using in complex human internal environments.
Key Words: nanomotors; gold nanoparticles; urease-powered; enhanced diffusion; biocompatibility
目录
摘要 I
Abstract II
第一章 绪论 1
1.1. 引言 1
1.2. 构筑纳米马达的材料种类 1
1.2.1. 介孔二氧化硅纳米颗粒 2
1.2.2. 纳米金 3
1.2.3. 高分子聚合物 4
1.3. 生物酶驱动纳米马达的种类 6
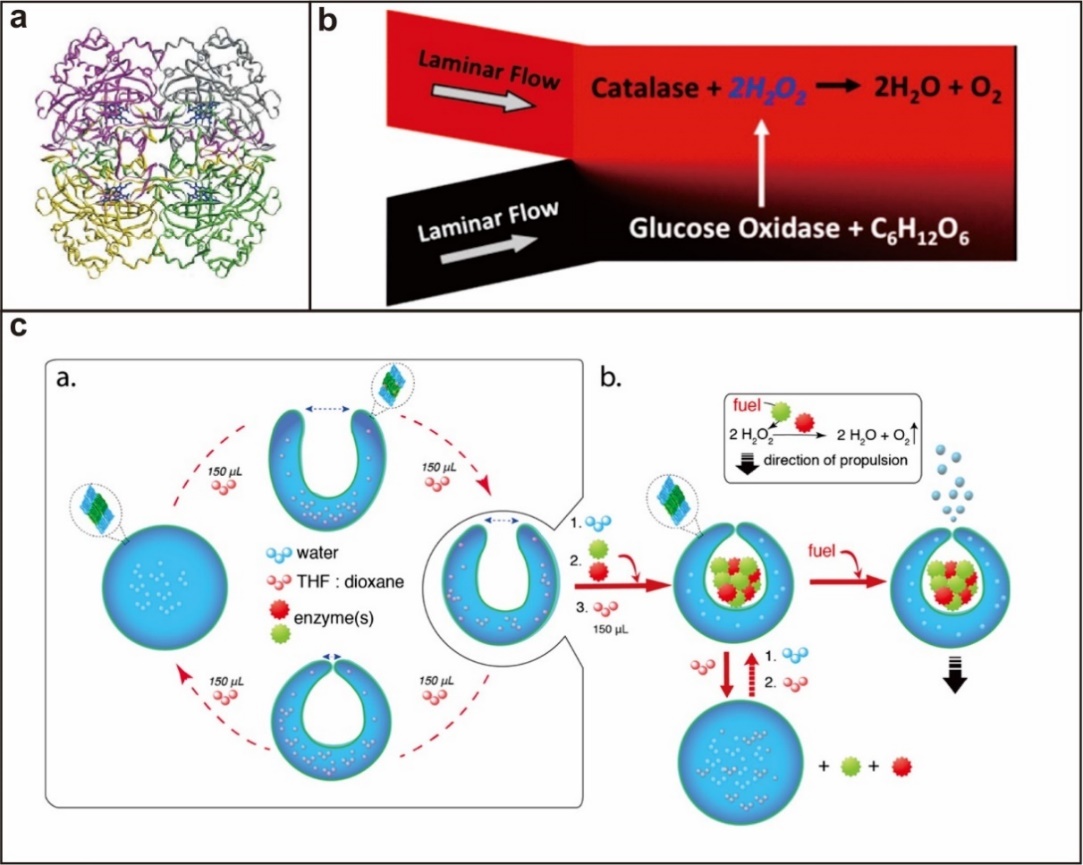
1.3.1. 过氧化氢酶驱动型 6
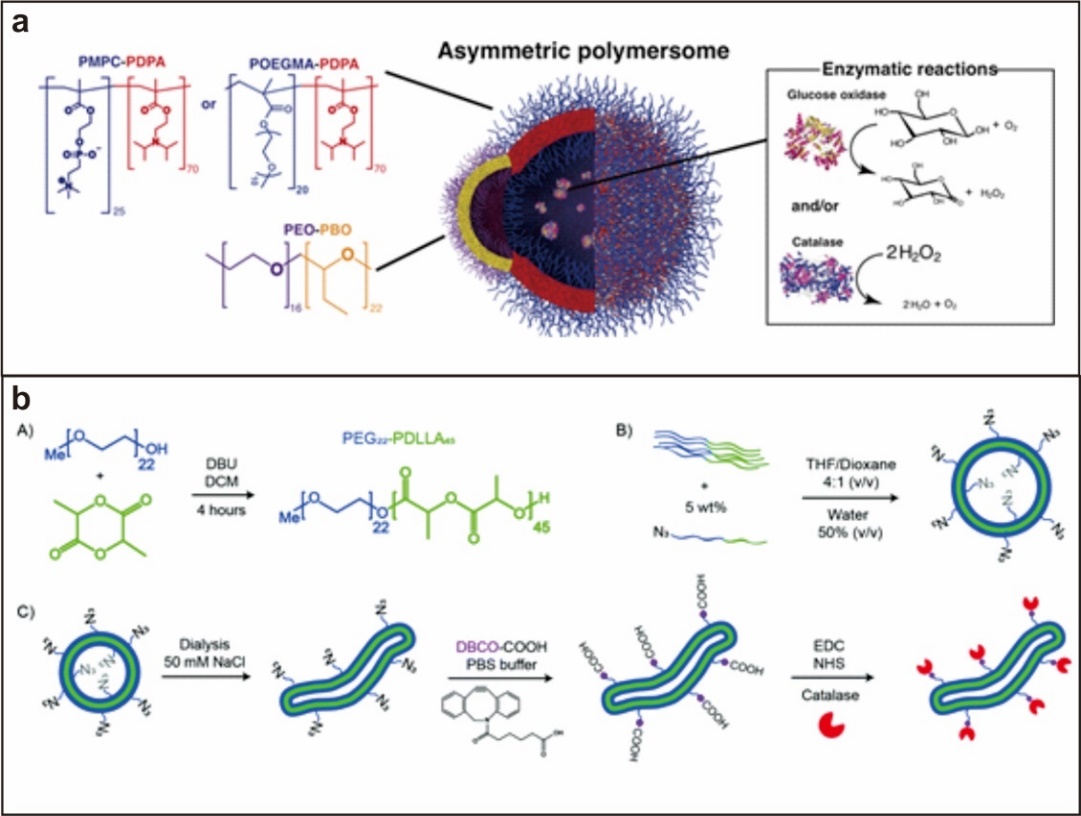
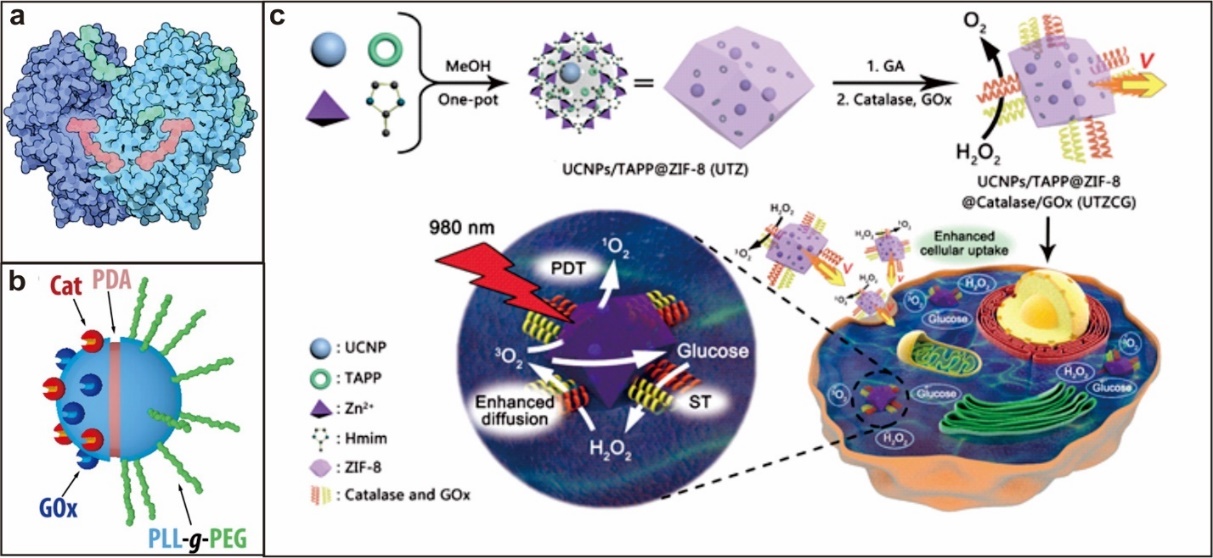
1.3.2. 葡萄糖氧化酶驱动型 7
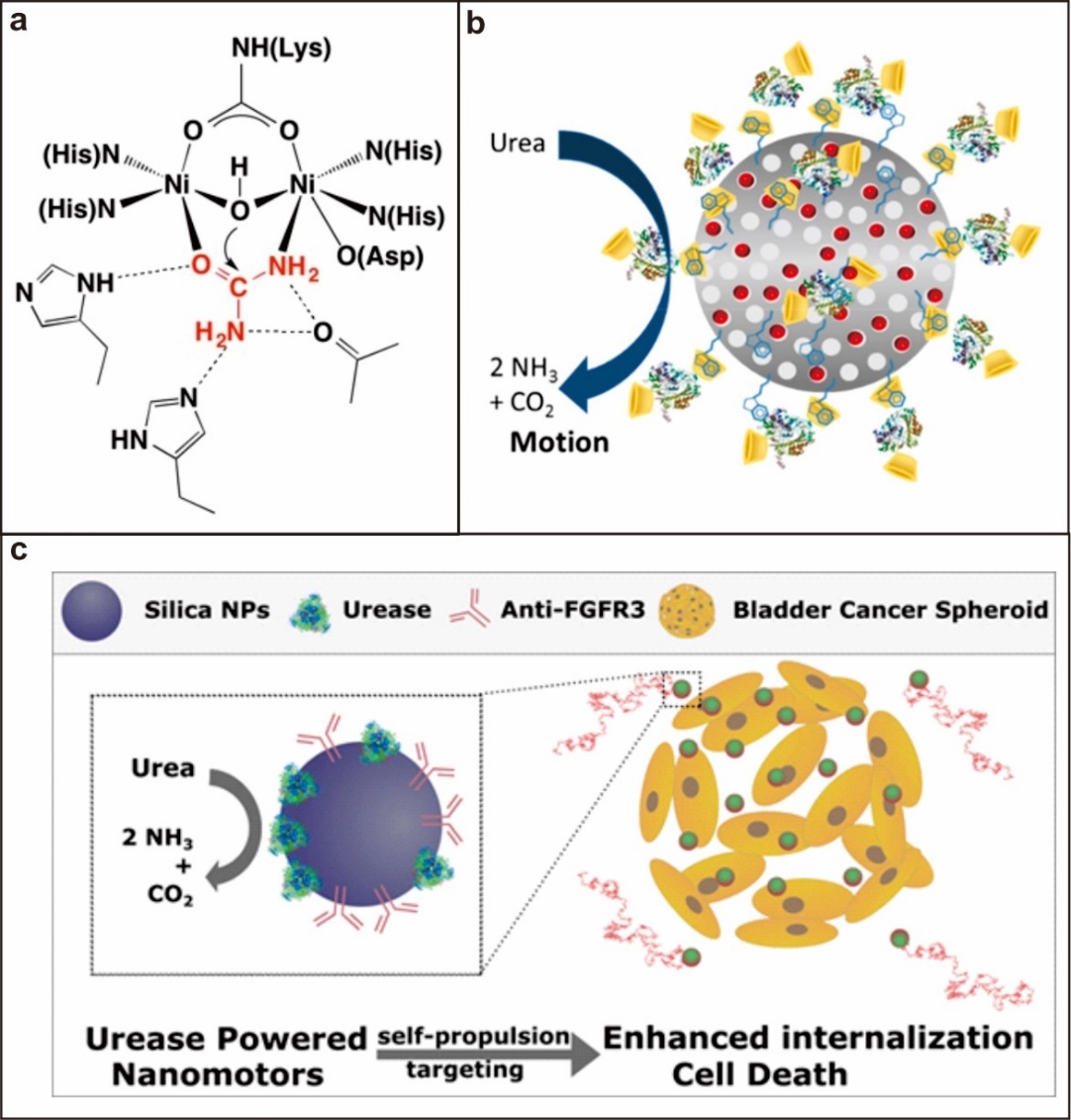
1.3.3. 脲酶驱动型 8
1.4. 纳米马达的运动表征方法 10
1.4.1. 动态光散射技术 10
1.4.2. 荧光相关光谱 12
1.4.3. 纳米粒子跟踪分析 13
1.4.4. 纳米冲击伏安法 14
1.5. 本论文研究目的及研究内容 15
第二章 脲酶驱动纳米金马达的制备及其运动性能表征 17
2.1. 引言 17
2.2. 实验部分 17
2.2.1. 实验试剂和仪器设备 17
2.2.2. 脲酶驱动纳米金马达的制备方法 19
2.2.3. 样品的测试与表征方法 22
2.3. 结果与讨论 24
2.3.1. 纳米金的成分、形貌及样品浓度表征 24
2.3.2. BSPP-Au的成分、形貌表征 26
2.3.3. SiO2@Au的形貌表征 27
2.3.4. PEG@Au不对称结构的表征 28
2.3.5. Urease@Au@PEG的不对称结构的验证 28
2.3.6. 马达运动性能表征 30
2.4. 本章小结 35
第三章 结论与展望 37
参考文献 38
致谢 43
第一章 绪论
1.1. 引言
微纳米马达(Micro-/nanomotors)是一类能够将周围环境中其它形式的能量(如化学能、热能、电场能及光能等)转化为动能的微纳米器件,[1]而纳米马达则是尺寸在纳米尺度的马达。纳米马达在自然生物中有许多实例,例如,人体内的驱动蛋白和肌球蛋白就是一类典型的马达,它们分别负责在细胞内的货物运输和肌肉运动,从ATP到ADP的转化中获取能量;许多类型的细菌(如大肠杆菌)也是微型马达,通过旋转附着在身上的鞭毛或纤毛推动自身实现运动。与之相比,人造马达的自驱动机理的本质在于构建一个非对称场(如局部电场、浓度梯度、表面张力梯度),打破马达的静力平衡,使马达产生自主运动,由于这种独特的自主运动性能,微纳米马达在生物传感、主动给药及微创手术等生物医药领域具有广泛的应用前景。[2]
马达作为药物载体在人体中的运输过程大致可以分为在血管中的运输、穿透血管壁进入组织间隙、在组织间隙中的运动和进入病变细胞四个步骤,现代科学家们认为通过非特异性内吞作用被内吞入非吞噬细胞的纳米颗粒上限大小为150 nm[3]。过去十年中合成马达的大小大多集中于微米尺寸,如Mallouk等提出的以过氧化氢为燃料的Pt/Au马达,其尺寸约为2 µm,这导致多数已合成出的马达无法应用于人体内环境,因此如何制备更小尺寸的马达成为如今的研究重点之一。
请支付后下载全文,论文总字数:39605字
相关图片展示: