甲酸锂基water-in-salt电解液的制备及其电性能研究毕业论文
2020-04-15 17:41:37
摘 要
当今世界人们的日常生活与手机、电脑紧密联系在一起,锂离子电池在给移动电子设备带来革命性变化的同时,也逐渐显示出其内在的弊端。随着锂离子电池在电子设备、电动汽车和电能储存等领域的广泛使用,对其安全性与成本增加的担忧也与日俱增。传统锂离子电池采用有机电解质,虽然在电化学窗口,能量密度等方面达到了预期要求,然而有机电解质的易燃性导致锂离子电池的安全性受到质疑,其毒性、挥发性导致在组装过程中的需要采取合适的保护措施。采用金属锂作为负极组装电池可以大幅提高电池能量密度,但是锂枝晶的产生容易刺破隔膜,导致短路,后果极其严重。水电解质的高安全性逐渐受到人们的关注。
水系锂离子电解液的优点十分明显:1)容量与传统铅酸电池,镍镉电池相当,但在制备过程中却对环境几乎无污染2)水系电解液的离子电导率较高,可以保证锂离子的快速传输3)水系锂离子电池的制造过程要求相较于有机电解液体系较为宽松,有机电解液往往要求生产环境除水除氧。4)水系锂离子电解液通常使用LiNO3、Li2SO4、CH3COOLi等,价格较有机电解液体系便宜许多。但水系电解液并非没有缺点。主要问题在于:1)电化学窗口较窄,纯水的电化学窗口仅有1.23V,这也导致了水系锂离子电池的能量密度较低(lt;70Wh/Kg)2)锂嵌入的电化学与非电化学过程都十分复杂,且伴随有副反应。3)集流体在循环过程中易被腐蚀,4)容量衰减快并且库伦效率低,过高的电压会导致电极表面发生析氢,造成安全问题,即使是微量的氢,在循环过程中也会严重破坏电极结构。这些问题限制了水系锂离子电池的广泛应用。
近年来,水盐电解质(water-in-salt,WIS)作为一种新型电解质,成功克服了水系锂离子电池电化学窗口狭窄的问题,据相关文献报道,其电化学窗口提升到了3.0V左右。WIS电解质定义为溶解盐的含量超过水的含量。最早的WIS电解质采用的是LiTFSI,但是由于LiTFSI价格极其昂贵且本身具有毒性,必须寻找更合适的电解质代替。
同时WIS锂离子电池具有一下问题有待解决。(1) 对应Water-in-salt电解液体系下的水系电池体系正负极材料筛选可以进一步优化; (2)Water-in-salt电解质的安全性能仍有待于进一步全面测试。
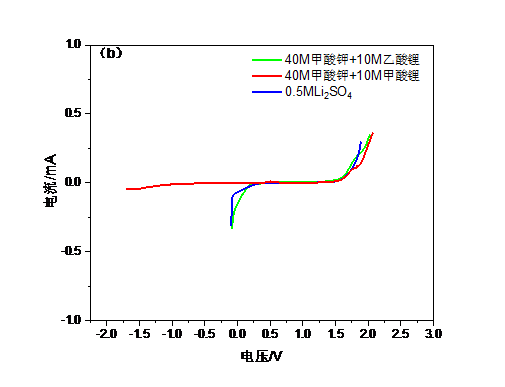
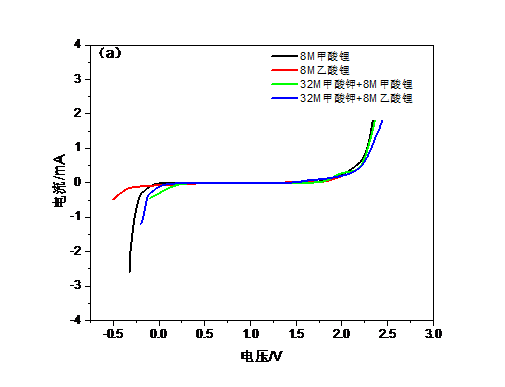
本文尝试采用甲酸钾,甲酸锂,乙酸锂等盐组合代替LiTFSI,采用双盐体系提高电解质浓度,以实现更宽的电化学窗口,同时使用0.5M Li2SO4这种常见的水系电解液作为对比。由于锂盐缺乏足够高的水溶性因而选择加入钾盐,以共溶盐的形式实现高浓度WIS体系。钾盐的加入可以起到拓展电化学窗口的作用,甲酸钾与锂盐结合能使电解质与传统电极材料兼容的同时保持低成本且无害。当电解液浓度逐渐靠近极限时,不同离子的溶剂化壳层会强烈地相互渗透,也就是说锂离子的溶剂化壳层中不仅有水分子还会存在阴离子,进而导致电子密度的增加。此时水分子间的氢键发生断裂整个系统可被认为是接近理想的WIS系统。经测试40M甲酸钾 10M甲酸锂的组合达到了最大的电化学窗口,大约2.7V,而0.5M Li2SO4电解液的电化学窗口仅有约1.7V。而WIS体系的电导率也比Li2SO4高,意味着锂离子的传输更快捷。WIS电解质的电化学性能通过LiFePO4正极材料进行了评价,LiFePO4/WIS/活性炭电池的容量为约130mAh g-1, 与传统电解质相差不多,且表现出较好的循环稳定性。该电解质制备简单,性能优良,将大幅提高大容量锂离子电池安全可靠性和电化学性能。
关键词:锂离子电池;高浓度电解质;甲酸钾(CHKO2) ;甲酸锂(CHLiO2)
Preparation and Electrical Properties of Lithium Formate Based Water-in-salt Electrolyte
Abstract
Nowadays,people's daily life is closely related to mobile phones and computers. Lithium ion batteries bring revolutionary changes to mobile electronic devices, but at the same time, they gradually show their inherent disadvantages. Concerns about the safety and cost of lithium-ion batteries are growing as they are used in electronics, electric cars and power storage. Traditional lithium ion batteries use organic electrolyte, although the electrochemical window, energy density and other aspects meet the expected requirements, but the flammability of organic electrolyte leads to the safety of lithium ion battery is questioned, its toxicity, volatility in the assembly process need to take appropriate protective measures. Lithium metal as a negative electrode assembly battery can greatly improve the battery energy density, but lithium dendrite production is easy to puncture the diaphragm, resulting in short circuit, the consequences are very serious. The safety of water electrolyte has been paid more and more attention.Water system of lithium ion electrolyte advantages are obvious: 1) the capacity and the traditional lead-acid batteries, nickel cadmium battery quite, but almost no pollution to the environment in the process of preparation of 2) drainage higher ionic conductivity of electrolyte, can guarantee the lithium ion the rapid transmission of 3) water system of lithium ion battery manufacturing process requirements compared with organic electrolytic liquid is looser, organic electrolyte often require production environment water oxygen.But water electrolytes are not without their drawbacks. The main problems are as follows: 1) the electrochemical window is narrow, and the electrochemical window of pure water is only 1.23v, which also leads to the low energy density of the water-system lithium ion battery (lt;70Wh/Kg); 3) the collecting fluid is easy to be corroded in the circulation process; 4) the capacity decays rapidly and the coulomb efficiency is low. The excessive voltage will lead to hydrogen evolution on the electrode surface, causing safety problems. Even the trace amount of hydrogen will seriously damage the electrode structure in the circulation process. These problems limit the wide application of water lithium ion batteries.Recently, water-salt electrolyte (WIS), as a new type of electrolyte, has successfully overcome the problem of narrow electrochemical window of lithium ion battery in water system. According to relevant literature reports, its electrochemical window has been improved to about 3.0v. WIS electrolytes are defined as the amount of dissolved salt in excess of the amount of water. The earliest WIS electrolyte used LiTFSI, but because LiTFSI is extremely expensive and toxic, a more suitable electrolyte must be found to replace it.Meanwhile, WIS lithium ion battery has the following problems to be solved. (1) the selection of anode and cathode materials for the Water battery system corresponding to the water-in-salt electrolyte system can be further optimized; (2) the safety performance of water-in-salt electrolytes remains to be fully tested.In this paper, potassium formate, lithium formate, lithium acetate and other salt combinations were used to replace LiTFSI. Double salt system was adopted to improve the electrolyte concentration to achieve a wider electrochemical window. At the same time, 0.5m Li2SO4, a common electrolyte of water system, was used for comparison. Due to the lack of water solubility of lithium salt, the potassium salt was added to achieve the high concentration WIS system in the form of co-dissolved salt. The addition of potassium salt can play a role in expanding the electrochemical window, and the combination of potassium formate and lithium salt can make the electrolyte compatible with traditional electrode materials while keeping low cost and harmless. When the concentration of electrolyte gradually approaches the limit, the solvated shells of different ions will strongly permeate each other, that is to say, the solvated shells of lithium ions will contain not only water molecules but also anions, which will lead to the increase of electron density. At this time, the hydrogen bond between water molecules breaks, and the whole system can be considered as an ideal WIS system. The combination of 40M potassium formate and 10M lithium formate reached the maximum electrochemical window, about 2.7v, while the electrochemical window of 0.5m Li2SO4 electrolyte was only about 1.8v. The conductivity of WIS system is also higher than that of Li2SO4, which means the transmission of lithium ions is faster. The electrochemical performance of WIS electrolyte was evaluated by LiFePO4 positive electrode material. LiFePO4/WIS/ activated carbon battery has a capacity of 120 mAh g-1, which is similar to that of traditional electrolyte, and shows better cycling stability. The simple preparation and excellent performance of the electrolyte will greatly improve the safety, reliability and electrochemical performance of large-capacity lithium ion batteries.
Key Words: lithium ion battery; High concentration electrolyte; Potassium formate (CHKO2); Lithium formate (CHLiO2)
目录
摘要 I
第一章 绪论 1
1.1引言 1
1.2 锂离子电池简介 3
1.2.1锂离子电池发展概况 3
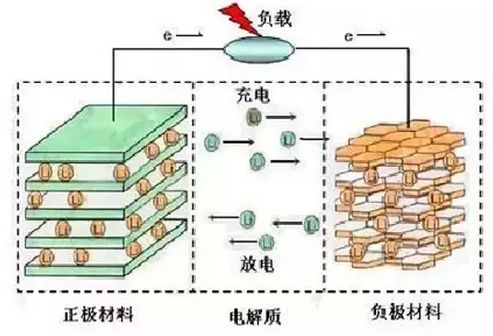
1.2.2 锂离子电池工作原理 4
1.2.3 水系锂离子电池正极材料 4
1.2.4 水系锂离子电池负极材料 5
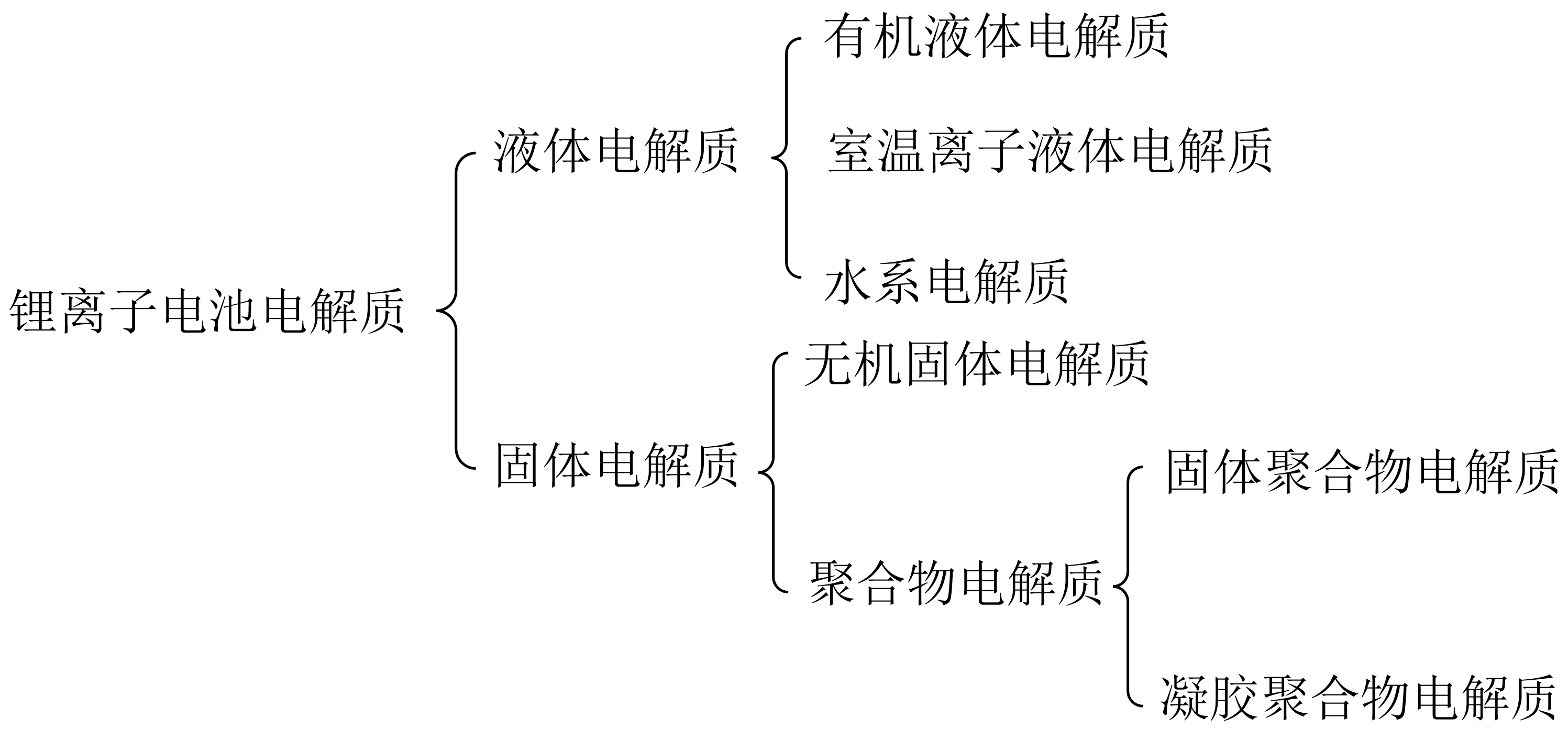
1.2.5 水系锂离子电池电解质 5
1.3 Water-in-Salt电解质简介 5
1.3.1 Water-in-Salt电解质发展概况 6
1.3.2 锂离子电池电解质分类 6
1.3.3 Water-in-salt电解质制备方法 7
1.4 本论文的研究目的和主要内容 7
1.4.1 研究目的 7
1.4.2 研究内容 7
第二章 实验材料与研究方法 9
2.1实验试剂 9
2.2实验仪器 10
2.3电化学性能测试 11
2.3.1离子电导率测试 11
2.3.2线性扫描伏安测试 11
2.3.3循环伏安测试 11
2.3.4恒流充放电测试 11
第三章 甲酸锂基Water-in-Salt电解液 12
3.1引言 12
3.2甲酸锂基water-in-salt电解液的制备 13
3.3电极制备 13
3.4甲酸锂基water-in-salt电解液的电化学性质 14
3.4.1离子电导率 14
3.4.2电化学窗口 15
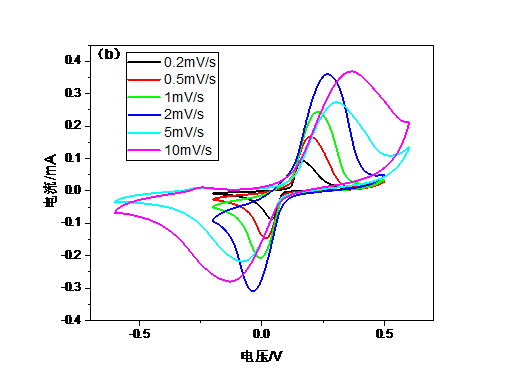
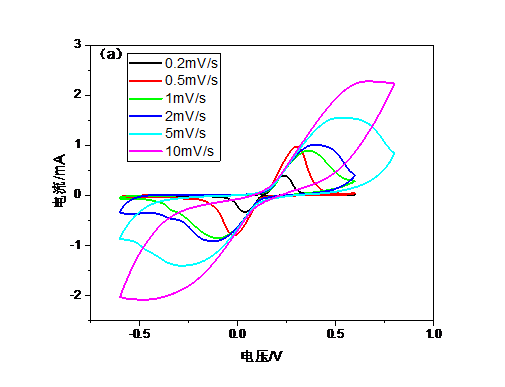
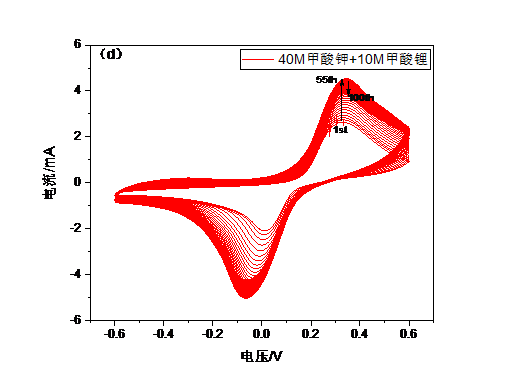
3.4.3 循环伏安曲线 15
3.4.4充放电曲线 17
3.4.5循环性能与倍率性能 18
第四章 结论与展望 20
参考文献 21
致谢 25
第一章 绪论
1.1引言
进入21世纪以来人类对于能源的需求愈来愈大,20世纪70,80年代发生了石油危机,这时人们逐渐意识到不能单一地依靠化石能源,尤其是石油[1]。化石能源的储量是有限的,经过长期地开采使用其储量在可预见的未来就会被全部消耗。据有关部门预测,世界范围内已探明的石油,天然气和煤炭分别还可以供人类使用50.2年、52.6年和134年。另外,化石能源的使用还造成了极其严重的环境污染。目前全球范围内汽车保有量已远超10亿辆,每天向大气中排放数十亿吨二氧化碳,二氧化硫。全球变暖,酸雨等问题已到了亟待解决的地步。不少国家的科学家经过多年的努力,找到了一些清洁替代能源,比如风能,太阳能,潮汐能。对化石能源的使用限制也与日俱增,挪威宣布2035年前禁止销售化石燃料汽车。显然汽车行业的转型升级已刻不容缓[2]。
以上是毕业论文大纲或资料介绍,该课题完整毕业论文、开题报告、任务书、程序设计、图纸设计等资料请添加微信获取,微信号:bysjorg。
相关图片展示:
您可能感兴趣的文章
- 激光作用下ZrNiSn合金热电材料组成、结构和性能的演化规律开题报告
- 原位生长于碳纤维表面的钒氧化物柔性电极制备开题报告
- 锂硫电池用TixOy-S/HGs复合材料的制备与性能开题报告
- MnO2纳米片修饰ZnO纳米棒阵列的气敏性能研究开题报告
- 基于三维碳基孔结构和电解质协同优化的微型超级电容器文献综述
- 基于C-MEMS工艺的微型混合锂离子电容器构筑及性能开题报告
- 多孔碳负载钼基纳米材料作为高性能析氢电催化剂文献综述
- Cu掺杂ZnxCd1-xS纳米晶的制备与性能研究开题报告
- 用于光伏的III-V族半导体低成本生长外文翻译资料
- 太阳能电池中的GaSb / InGaAs 量子点阱混合结构有源区外文翻译资料




