盐酸改性天然锰氧化物去除双酚A机理研究毕业论文
2020-04-18 20:06:29
摘 要
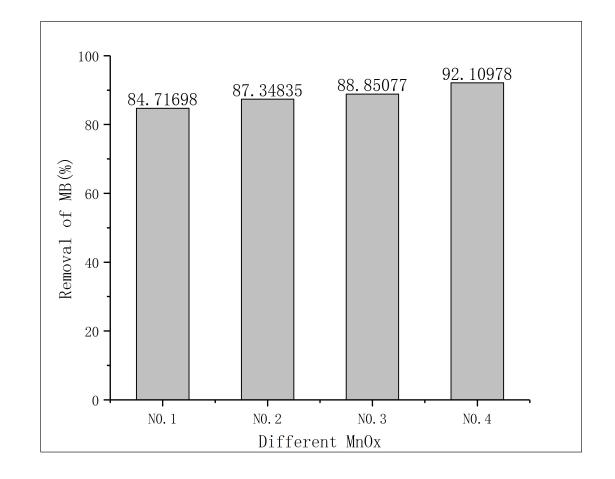
本文利用改性天然锰氧化物去除双酚A废水,通过筛选不同的改性条件选出最优改性条件,并以此改性条件下改性的锰氧化物为原材料,对反应环境中pH值、双酚A初始浓度变化、和投加改性锰矿石的量3种影响因素对于反应结果中双酚A降解效果的影响。实验结果表明;用5mol/L盐酸振荡3小时的改性条件在去除双酚A时1小时去除率就达到了92.1%,这组处理效果最好
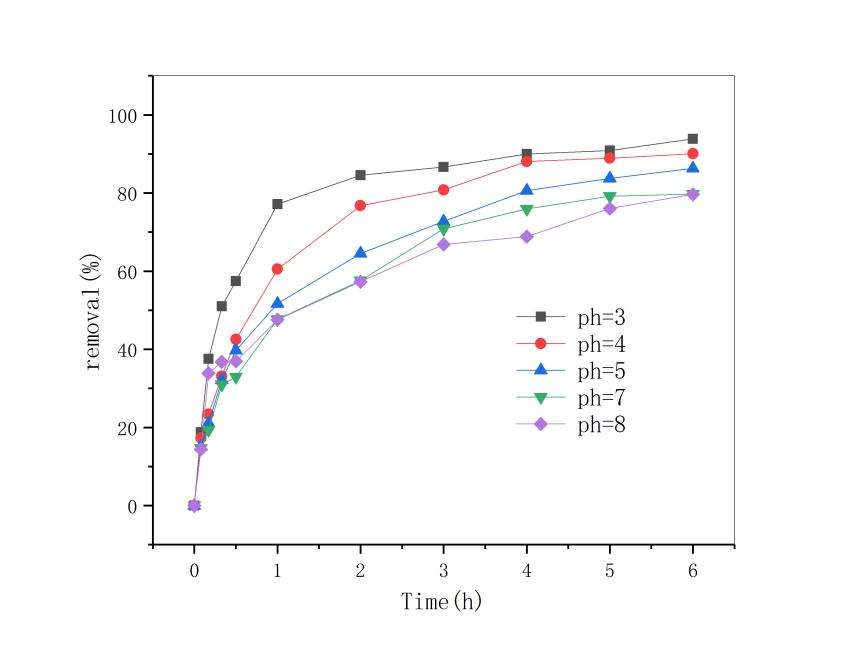
用此改性条件下的锰矿石做后续实验,随着反应体系pH值降低,双酚A的去除率明显上升。当反应pH=3时,反应6小时后,双酚A的去除率达到93.86%,;当天然锰矿石的投加量为5g/L时及双酚A初始浓度为10mg/L时,双酚A去除率在反应3h后全都达到了95% ;在控制pH,锰矿石投加量不变条件下,筛选出污染物浓度为5mol/L污染物去除 速率最快。因此,改性锰矿石投加量越多及双酚A初始浓度越低,去除效果就越好。最后对3种影响因素综合分析,得到了最优的反应条件即反应体系pH=3,5mol/L盐酸振荡3小时处理过的矿石投加量为5g/L,双酚A初始浓度为5mg/L时双酚A的降解效果比较好。通过对3种影响因子与双酚A去除率之间进行动力学方程拟合发现,三者均符合一级动力学方程,且拟合度均超过0.9。
关键词:双酚A 改性锰矿石 动力学 盐酸
Study on degradation mechanism of bisphenol a by modified manganese ore
In this research, natural manganese ore was used to remove bisphenol A wastewater, and the optimal modification condition was selected by screening different modification conditions. The modified manganese ore under this condition was used as raw material to explore the influence of three influencing factors, namely, pH of reaction system, initial concentration of bisphenol A, and addition amount of manganese ore, on the removal rate of bisphenol A. The experimental results showed that the removal rate of bisphenol A reached 92.1% within 1 hour when the modified condition of 5mol/L hydrochloric acid was oscillated for 3 hours, and the treatment effect was the best in this group. As the pH value of the reaction system decreased, the removal rate of bisphenol A increased obviously. When pH=3, the removal rate of reactive blue reached 93.86% after 6 hours of reaction. When the dosage of natural manganese ore was 5g/L and the initial concentration of bisphenol A was 10mg/L, the removal rate of bisphenol A reached 95% after 3h of reaction. Under the condition of controlling pH and constant addition of manganese ore, the pollutant concentration of 5mol/L was selected as the fastest pollutant removal rate. Therefore, the more the amount of modified manganese ore is added and the lower the initial concentration of bisphenol A is, the better the removal effect will be. Finally, by comprehensive analysis of the three influencing factors, the optimal reaction conditions were obtained, that is, the reaction system pH=3, 5mol/L of hydrochloric acid was oscillated for 3 hours, the dosage of treated ores was 5g/L, and the degradation effect of bisphenol A was better when the initial concentration of bisphenol A was 5mg/L. By fitting the kinetic equation between the three influencing factors and the removal rate of bisphenol A, it was found that all the three factors were in line with the first-order kinetic equation, and the fitting degree was over 0.9.
Keywords: bisphenol A ; Modified manganese ore ;dynamic ;hydrochloric acid
目 录
摘要 I
Abstract II
第一章 绪论 1
1.1 研究背景 1
1.2塑料污染的概述 1
1.2.1塑料的性质和来源 1
1.2.2塑料的危害 1
1.3选题依据 2
1.3.1双酚A的性质 2
1.3.2双酚A的危害 3
1.3.3锰氧化物的性质 3
1.3.4锰氧化物的去除和降解作用 4
1.3.5 锰矿石的应用 4
1.3.6 盐酸的性质 4
1.4研究的目的及主要内容 5
第二章 实验材料及准备 6
2.1 实验仪器及准备材料 6
2.2实验材料及溶液配制 6
2.3实验分析方法 7
2.3.1 BPA浓度计算方法 7
2.3.2 BPA去除率计算方法 8
2.3.3 Mn2 浓度计算方法 8
2.4 实验步骤 9
2.4.1 改性条件的筛选 10
2.4.2 pH的影响 10
2.4.3 锰矿石投加量的影响 10
2.4.4 双酚A初始浓度的影响 10
第三章 实验结果及讨论 11
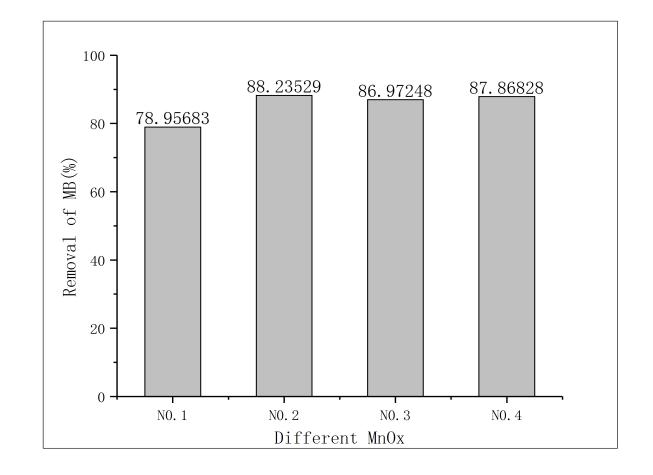
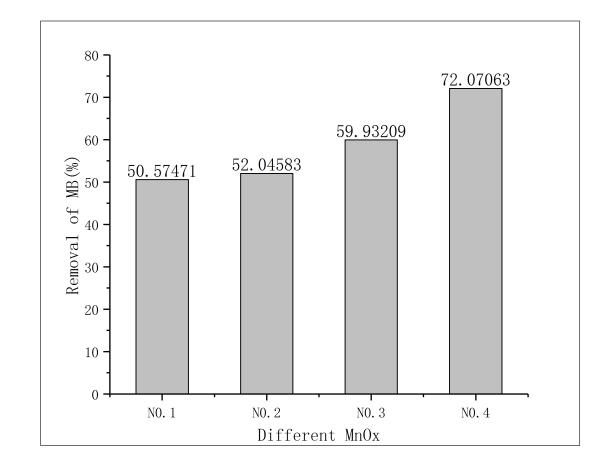
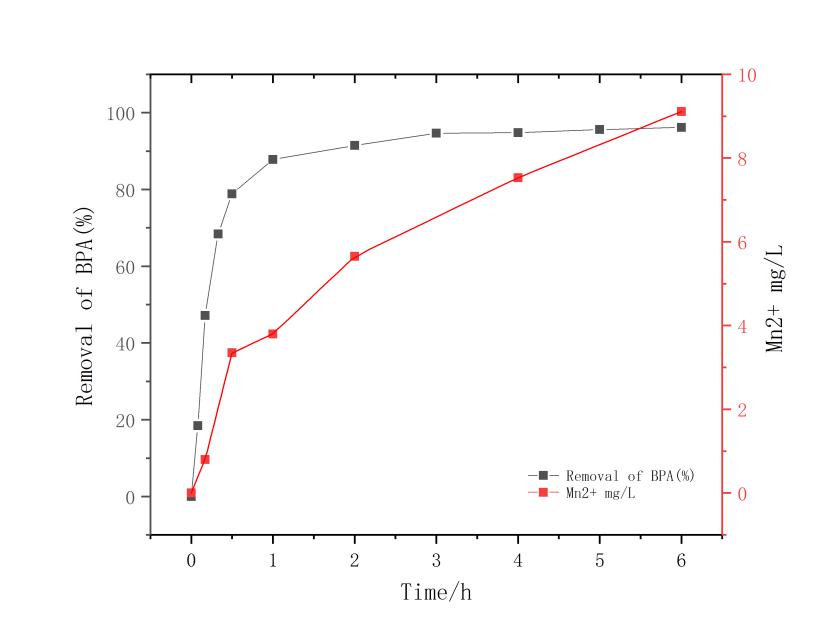
3.1改姓锰矿石的筛选 11
3.2 不同pH的影响 12
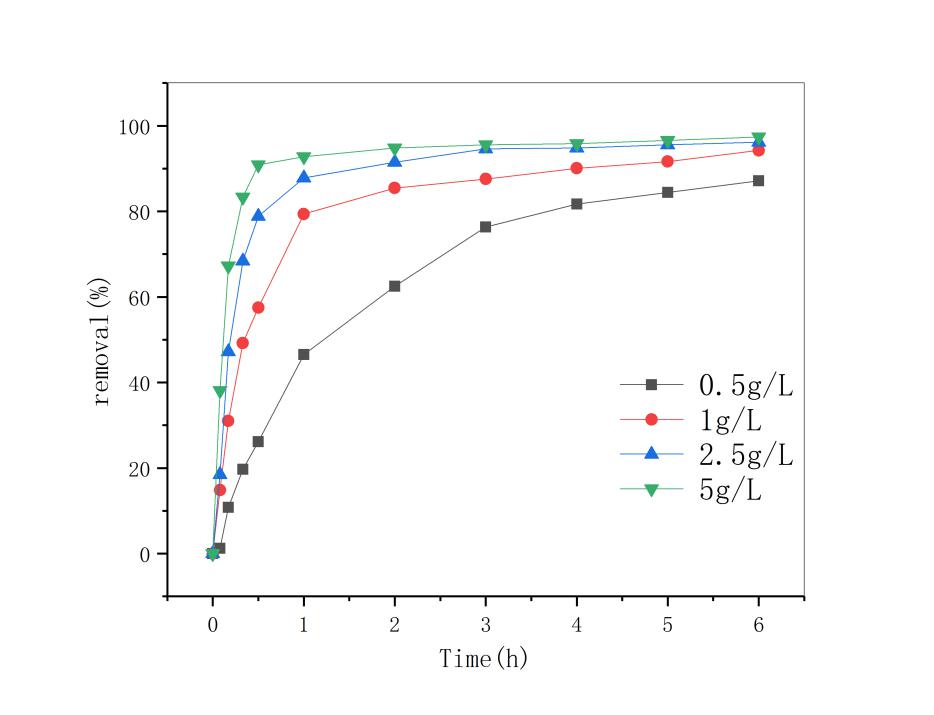
3.3 改姓锰矿石投加量的影响 13
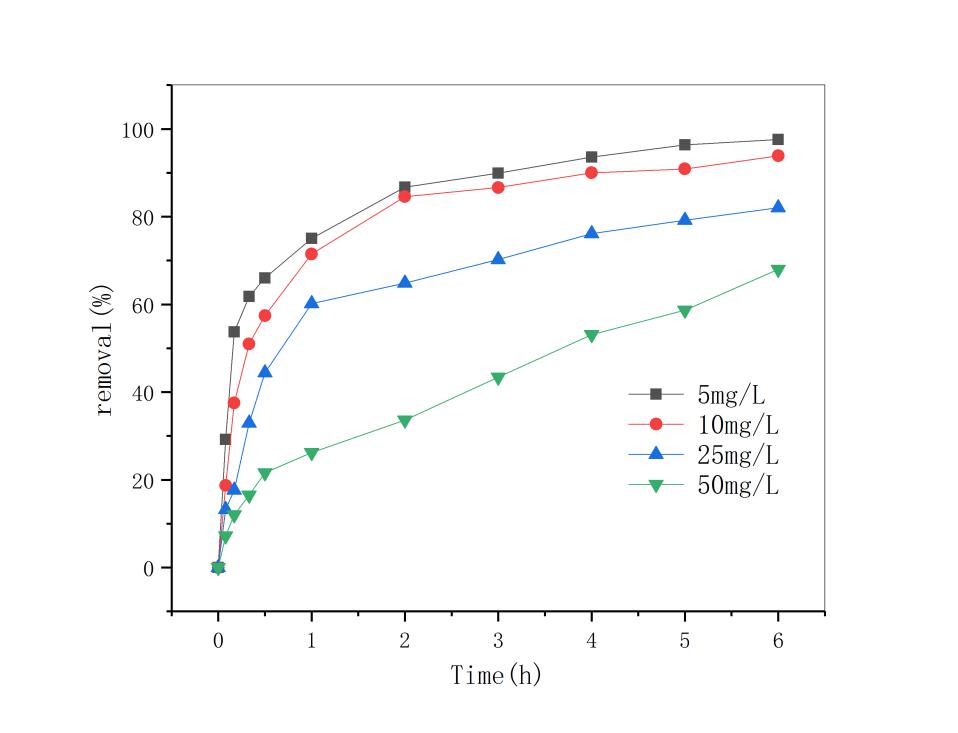
3.4 双酚A初始浓度的影响 13
3.5 BPA动力学分析 15
3.5.1动力学简介 15
3.5.2动力学结果 15
第四章 结论与展望 18
参考文献 19
致谢 21
第一章 绪论
1.1研究背景
在21世纪随着人类社会的进步,经济科学各方面领域的提高,为了更好的服务生活,为人们带来更好的经济效益,塑料被在全世界范围内大规模使用。然而随着塑料在生活中被广泛而大量的利用,其带来的危害也远远超出我们的想象。中国的塑料产量占全球的24.8%,是全世界最大的塑料出产国,而且我国出产的塑料还在以更快的速度上升。塑料制品被发明于20世纪,当时的人们因为其质量轻便,易塑形,可以完美替代其他金属材料,又具有很强的耐腐蚀能力而将之称为人类历史上最伟大的发明。然而人们未曾料到曾经给人们带来无数便利的塑料如今已经不知不觉成为人类的头号敌人。由于其难于自然降解,废旧塑料很难在环境中得到有效的处理,从来累积起来,带来严重的环境污染问题[1]。传统工艺对于塑料的处理具有很大的局限性,因此,找到有效处理废弃塑料的方法不仅有利于社会的发展,也能够为自然环境及人类提供安全保障。
1.2塑料污染的概述
以上是毕业论文大纲或资料介绍,该课题完整毕业论文、开题报告、任务书、程序设计、图纸设计等资料请添加微信获取,微信号:bysjorg。
相关图片展示: