合成EEP的高稳定性固体碱催化剂研究毕业论文
2020-06-16 20:37:17
摘 要
3-乙氧基丙酸乙酯(EEP)是一种低挥发性的醚酯溶剂,被广泛用于电子、清洗、涂料、印刷油墨及医药中间体合成等行业。目前工业上主要采用乙醇和丙烯酸乙酯加成制备EEP,这是典型的原子经济反应,但是该反应对催化剂的碱性要求较高。负载型的碱金属氧化物催化剂由于具有较为宽广的碱性范围以及制备方法简单等优点,得到了广泛的研究和应用,KNO3作为一种中性化合物,通过负载在合适的载体上,经过焙烧之后可以产生强碱性位,表现出优异的催化活性,是一种高效、经济的固体碱催化剂。
本论文采用具有笼状结构的低硅铝比分子筛NaA作为载体,但是由于KNO3的分解温度高(gt;700 oC),若焙烧不完全,会导致催化剂碱性低,催化反应速率慢。由此,本论文通过采用溶剂辅助分步焙烧(solvent-assisted stepwise calcination approach, SASC)的方法促进KNO3在低温下二次分解产生更多碱性位,并探索了其在丙烯酸乙酯和乙醇加成制备EEP中的工艺条件和催化剂的重复使用性能。结果表明,SASC法制备固体碱催化剂过程引入有机溶剂处理后,有机溶剂在N2氛围下碳化生成的C与未分解的KNO3发生氧化还原反应,促进KNO3的进一步分解,从而产生更多的碱性位。相对于常规等体积浸渍法制备的固体碱催化剂,采用SASC法可以明显提高催化剂的碱强度和碱量。但是相对易挥发的有机溶剂在低温下大部分会挥发而不是碳化,从而影响KNO3的分解量。K2O/NaA催化剂用于丙烯酸乙酯和乙醇的加成反应中,结果表明,15%K2O/NaA-S具有最高的催化活性,当反应温度为70 oC,乙醇和丙烯酸乙酯摩尔比为8:1,催化剂用量为丙烯酸乙酯的10 wt%时,丙烯酸乙酯的转化率为95.3%,EEP的选择性为99.1%。15%K2O/NaA-S重复使用4次后,丙烯酸乙酯的转化率依然保持在90%以上,EEP的选择性保持在99%左右,其原因在于活性组分K元素的流失量极少。XRF结果表明,催化剂经过4次重复使用之后K元素的流失量仅为6.27%。
关键词:固体碱 K2O 加成反应 重复使用性能 3-乙氧基丙酸乙酯
STUDY ON SOLID BASE WITH HIGH STABILITY FOR THE SYNTHSIS OF ETHYL 3-ETHOXYPROPIONATE
ABSTRACT
Ethyl 3-ethoxypropionate (EEP), a kind of ester-ether solvent with low volatility and viscosity, is widely believed as a possible substitute of conventional coatings. Besides, for its high solubility with most polymers, it also has been widely used in electronic, cleaning, printing and medical industries, etc. By far, most of commercial EEP comes from the additive reaction of ethanol to ethyl acrylate, which is a typical atom economic reaction. Nevertheless, it has high demands for the base strength of catalysts. Alkali metal oxide-loaded catalysts have been studied extensively due to its broading range of basicity and simple preparation methods. Especially, KNO3, as a kind of neutral salt, is widely used as precursor to generate basic sites after calcination. Hence, it is considered as an efficient and economical way to obtain the desired solid base.
Secondly, zeolite NaA with porous structure and negative charge of skeleton oxygen was applied as support. Nevertheless, the decomposition temperature of KNO3 is high (gt;700 oC). Direct calcination of KNO3 over NaA zeolite only generate catalyst with a minimal amount of K2O, which lead to the obtained catalysts only possessed weak basicity. Therefore a solvent-assisted stepwise calcation approach (SASC) was developed to further generate strong basicity on zeolite. Through pretreating by appropriate organic solvents, the base strength and concentration of catalysts increased significantly. The characterization of TG-MS revealed that the introduced solvents were firstly carbonized and generated carbon. Then the existence of reductive carbon facilitated the decomposition of KNO3 into K2O. But the volatile ones tended to escape from the system, not carbonize when heating under the N2 flow, which would affect the decomposition amount of KNO3. The catalytic activities were also evaluated via the addition reaction of ethyl acrylate and ethanol. Results showed that 15%K2O/NaA-S exhibited the highest catalytic activity (95.3% conversion of ethyl acrylate and 99.1% selectivity of EEP). To determine the reusability of the 15%K2O/NaA-S, the catalyst was reusable without much loss in activity for four cycles. XRF tests indicated that only 6.27% potassium leached after four cycles.
Key words: Solid base;K2O;Additive reaction;Reusability;Ethyl 3-ethoxypropionate
目 录
摘要 I
ABSTRACT II
第一章 文献综述 1
1.1 3-乙氧基丙酸乙酯的性质及用途 1
1.2 3-乙氧基丙酸乙酯合成研究进展 2
1.3 合成3-乙氧基丙酸乙酯的催化剂研究进展 3
1.4 负载型固体碱催化剂简介 7
1.5 本课题研究思路和研究内容 10
第二章 实验材料与实验方法 12
2.1 实验试剂及仪器 12
2.2 催化剂的表征方法 13
2.2.1 X射线衍射(XRD) 13
2.2.2 傅里叶变换红外光谱(FT-IR) 13
2.2.3 氮气吸附脱附(N2-Adsorption Desorption) 13
2.2.4 Hammett指示剂法 13
2.2.5 CO2程序升温脱附(CO2-TPD) 14
2.2.6 X射线荧光光谱分析(XRF) 14
2.2.7 热重-质谱连用(TG-MS) 14
2.3 实验方法 14
2.3.1 实验装置及流程 14
2.3.2 分析方法 15
第三章 溶剂辅助制备K2O/NaA催化合成3-乙氧基丙酸乙酯 18
3.1 实验方法 18
3.1.1 催化剂的制备 18
3.1.2 催化剂的表征 19
3.1.3 催化剂的活性评价 19
3.2 K2O/NaA催化剂表征 19
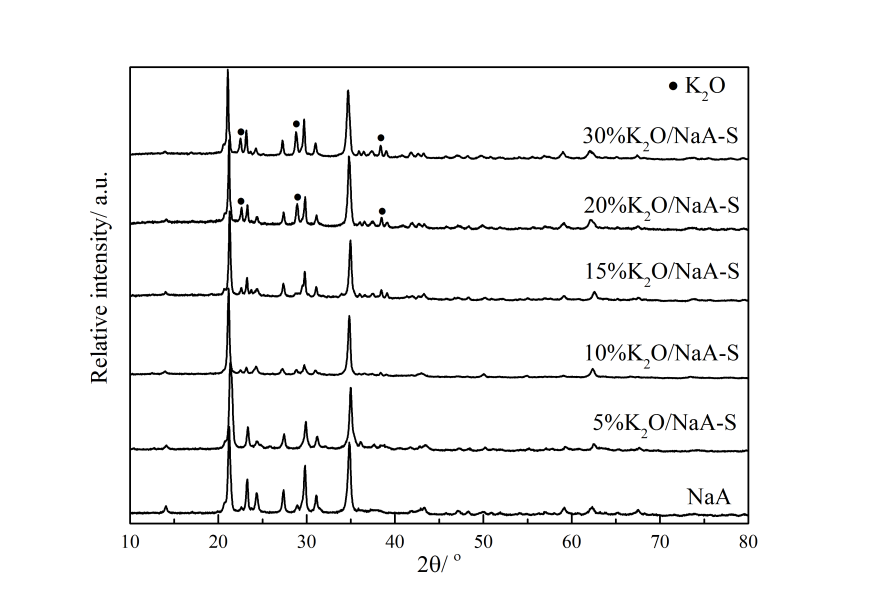
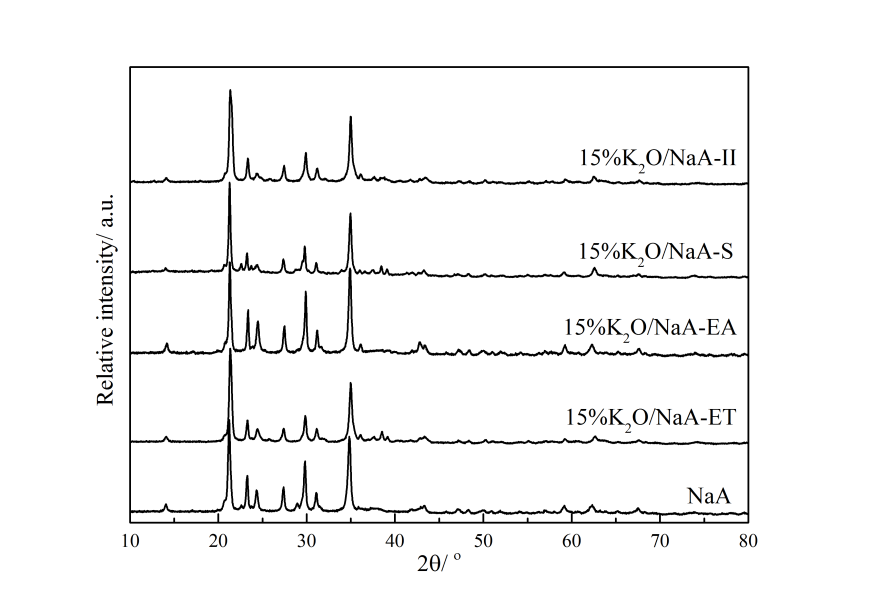
3.2.1 XRD表征 19
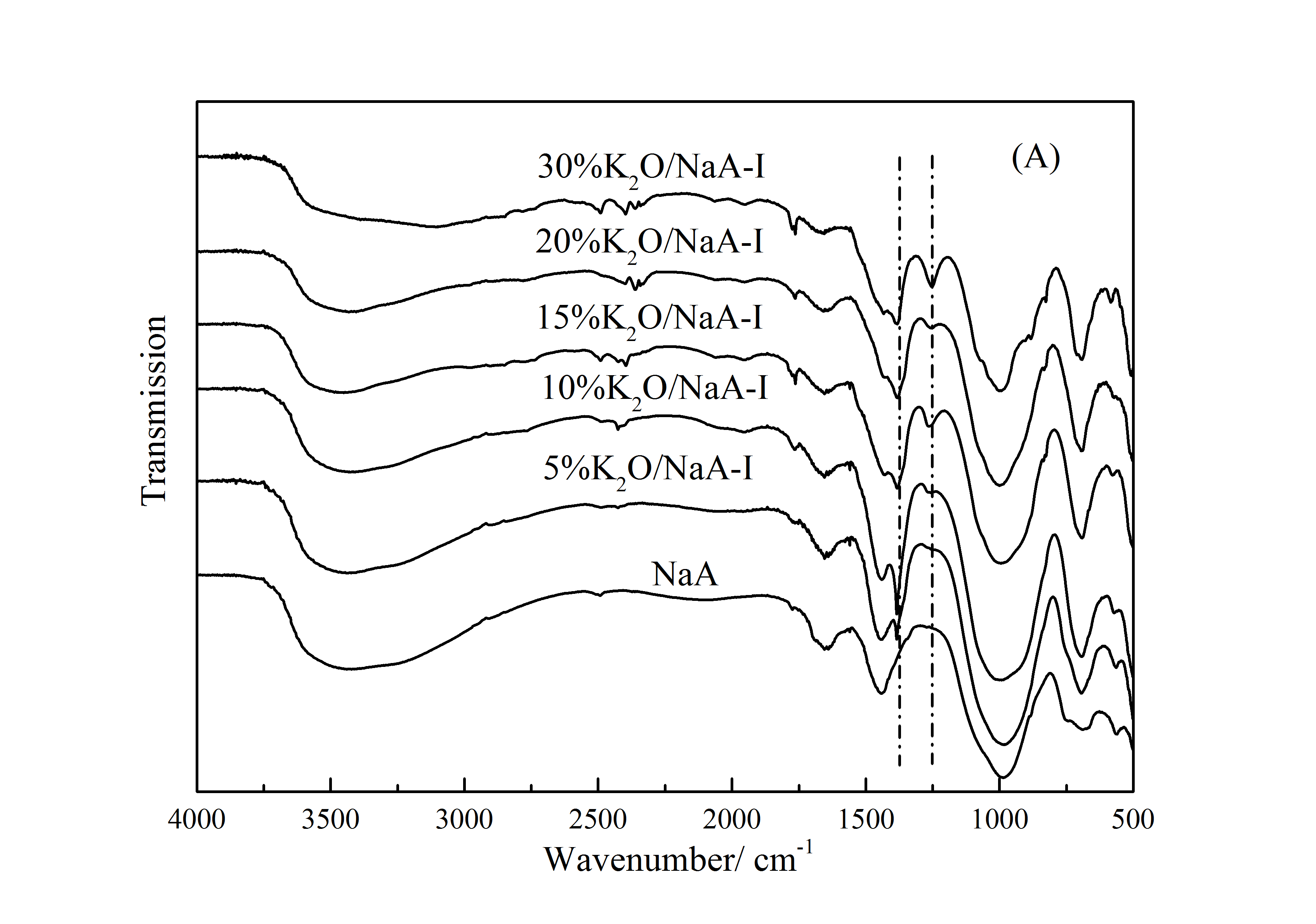
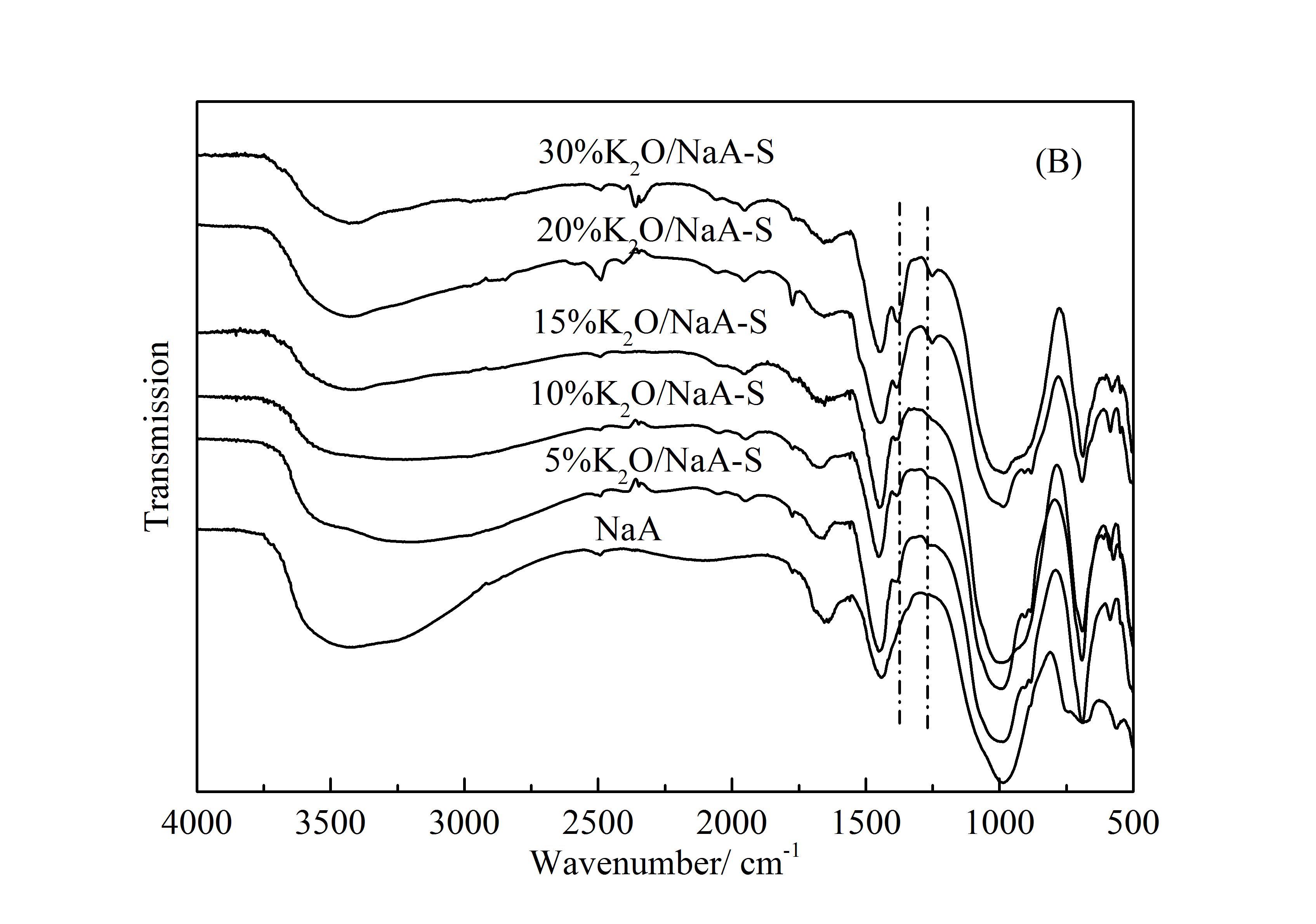
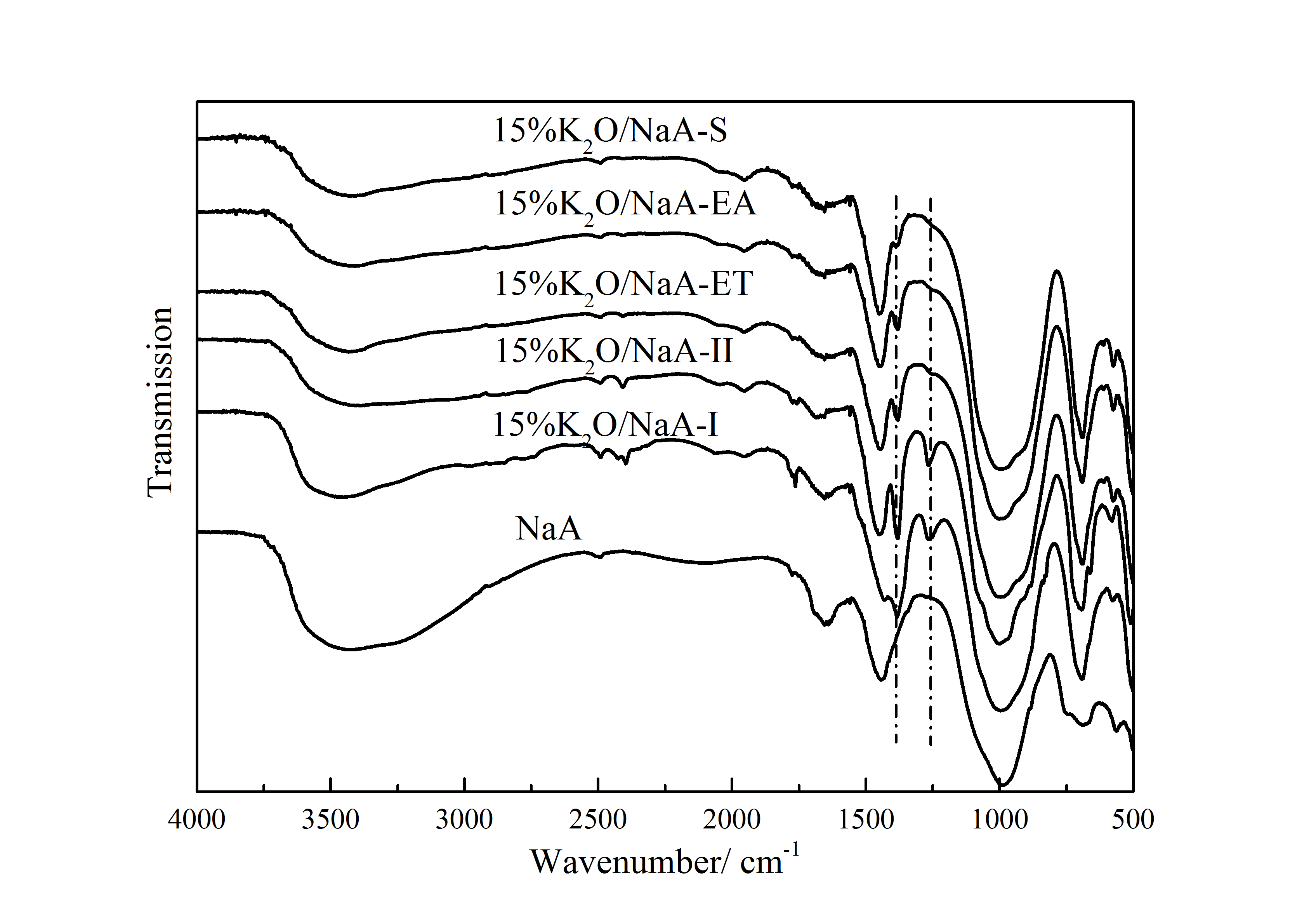
3.2.2 FT-IR表征 20
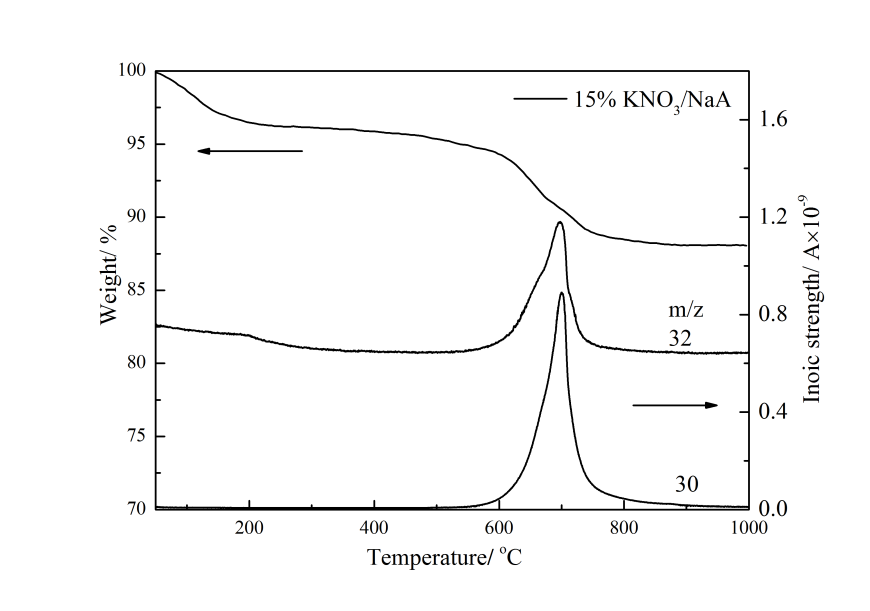
3.2.3 TG-MS表征 21
3.2.4 Hammett指示剂法 23
3.2.5 CO2-TPD表征 24
3.3 催化剂性能测试 25
3.3.1 不同催化剂对反应的影响 26
3.3.2 不同工艺条件对反应的影响 27
3.3.3 催化剂重复使用性能结果 29
第四章 结论 31
参考文献 32
致谢 36
第一章 文献综述
1.1 3-乙氧基丙酸乙酯的性质及用途
3-乙氧基丙酸乙酯英文名称为Ethyl 3-ethoxypropionate,简称EEP,化学式为: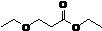 ,其主要的理化性质如表1-1所示。
,其主要的理化性质如表1-1所示。
表1-1 3-乙氧基丙酸乙酯的性质
名称 | 性质 | 名称 | 性质 |
分子式 | C7H14O3 | 沸点 | 166.2 oC |
分子量 | 146.2 | 相对密度 | 0.949 |
CAS号 | 763-69-9 | 闪点 | 52.2 oC |
性状 | 无色透明液体 | 折射率 | 1.405 |
熔点 | -75 oC | 相对蒸气密度 | 5.03 |
Table 1-1 The property of ethyl 3-ethoxypropionate
EEP是一种低挥发性的醚酯溶剂,由于其结构呈线性,分子中含有醚酯活性基团及丙酰基,EEP兼有了其他溶剂所不具备的一些性质,如热稳定性高,溶液粘度低,对很多聚合物具有较强的溶解性等,被广泛地应用于电子、清洗、涂料、印刷油墨及医药中间体合成等行业。
相关图片展示: