替卡格雷中间体四氢环戊二烯醇衍生物的合成毕业论文
2020-05-26 20:25:05
摘 要
论文以环戊二烯为原料,经过环氧化和叠氮化等多步反应合成四氢环戊二烯醇衍生物。讨论了投料比、温度、pH值、溶剂和缚酸剂对产率的影响。确定了环氧化第一步的最佳优化条件,反应温度在0-4℃之间,反应时间为2小时,溶剂为二氯甲烷,缚酸剂为碳酸钾。确定了环氧化第二步的最佳优化条件,反应温度在0-4℃之间,反应时间为24小时,溶剂为氯仿,缚酸剂为碳酸钾,投料比为1:1.1。分别用GC和1H-NMR对产物进行了鉴定。
关键词:环氧化反应; 过氧乙酸; 间氯过氧苯甲酸; 叠氮化钠;
ABSTRACT
The derivatives of tetrahydrocyclopentadienol was synthesized via two epoxidations of cyclopentadiene, followed by reaction with sodium azide. The reactant ratio, acid-binding agent, pH value and reaction temperature was discussed to improve the yield. The optimum conditions were determined. For the first step, the reaction temperature was within the range of 0-4℃, the reaction time was 2 hours, the solvent was methylene chloride and the acid-binding agent was potassium carbonate. For the second step, the reaction temperature was varied from 0-4℃, the reaction time was 24 hours, the solvent was chloroform, the acid-binding agent was potassium carbonate and the reactant ratio of the substrate and m-chloroperoxybenzoic acid was 1: 1.1. The products structure were proved by GC and 1H-NMR respectively.
Key Words: epoxidation reaction; peracetic acid; m-chloroperoxybenzoic acid; sodium azide;
目录
摘要……………………………………………………………………………………I
ABSTRACT………………………………………………………………………..Ⅱ
目录………………………………………………………………………………….Ⅲ
- 文献综述………………………………………………………………….1
- 前言………………………………………………………………………………1
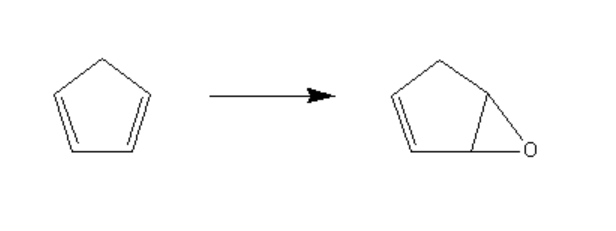
- 环戊二烯的环氧化………………………………………………………………3
1.2.1 6-氧杂-双环[3.1.0]己-2-烯的合成………………………………………4
- 3,7-二氧杂-三环[4.1.0.02,4]庚烷的合成………………………………...4
1.2.3 其它环氧化合成路线…………………………………………………..…5
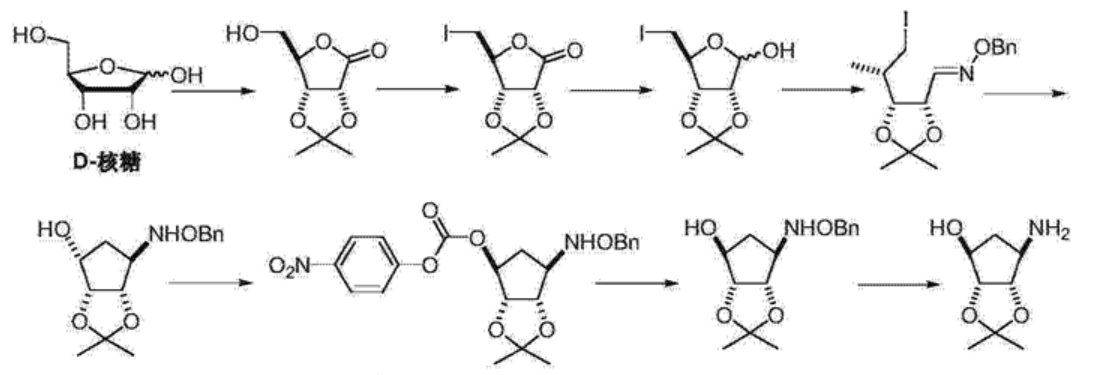
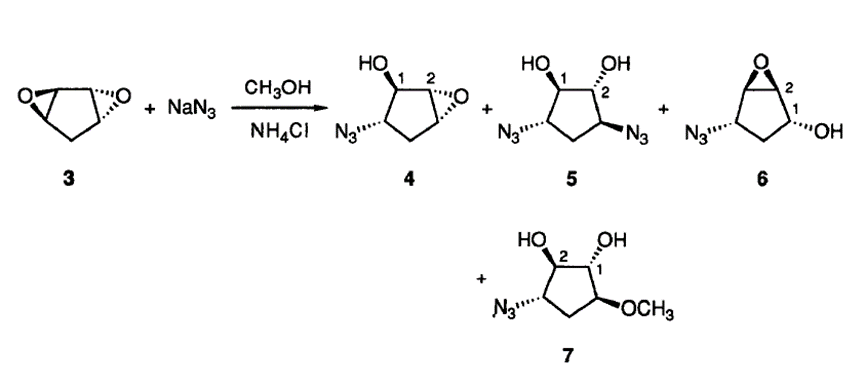
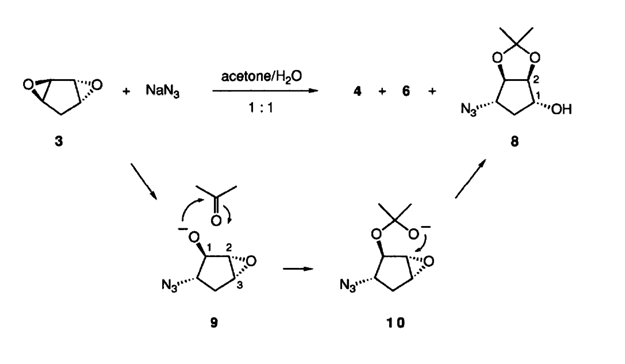
- 叠氮加成制备四氢环戊二烯醇衍生物…………………………………………7
- 选题的研究问题和解决途径…………………………………………………....8
第二章 6-氧杂-双环[3.1.0]己-2-烯的合成……………………………..........9
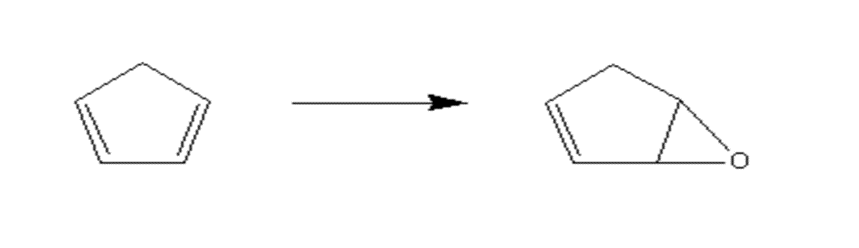
2.1 反应原理………………………………………………………………………….9
2.2 试剂与仪器……………………………………………………………………….9
2.2.1 试剂………………………………………………………………………..9
2.2.2 仪器………………………………………………………………………..9
2.3 实验部分………………………………………………………………………...10
2.3.1二聚环戊二烯的裂解…………………………………………………….10
2.3.2过氧乙酸的合成………………………………………………………….10
2.3.3 6-氧杂-双环[3.1.0]己-2-烯的制备……………………………………….10
2.3.4 提纯与精制………………………………………………………………11
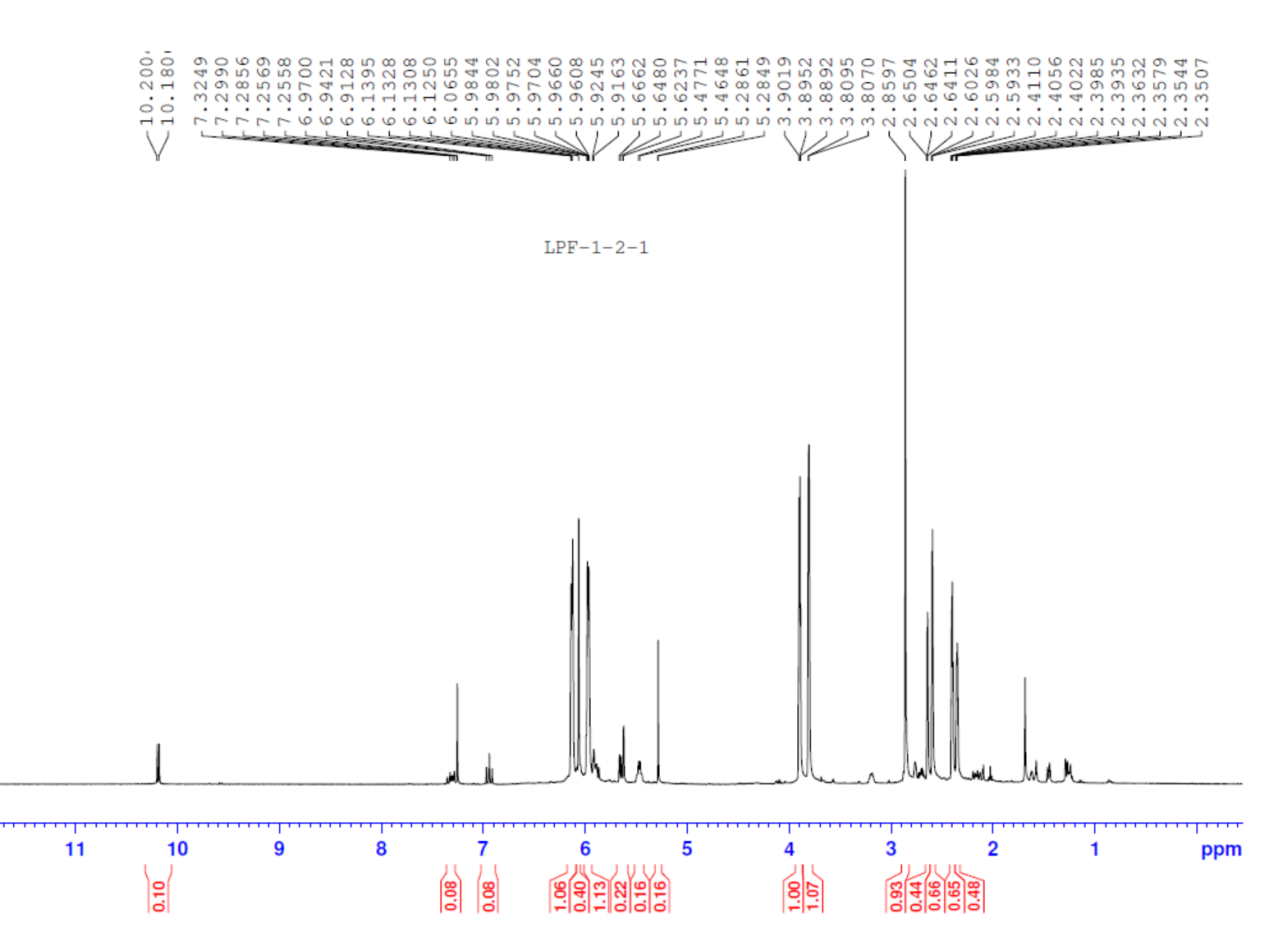
2.4 结构鉴定………………………………………………………………………...11
第三章 3,7-二氧杂-三环[4.1.0.02,4]庚烷的合成……………….………….13
3.1 反应原理………………………………………………………………………...13
3.2 试剂与仪器……………………………………………………………………...13
3.2.1 试剂………………………………………………………………………13
3.2.2 仪器………………………………………………………………………14
3.3 实验部分………………………………………………………………………...14
3.3.1 3,7-二氧杂-三环[4.1.0.02,4]庚烷的合成……………………………….14
3.3.2 溶剂的选择………………………………………………………………14
3.3.3 投料比的优化……………………………………………………………15
3.3.4 缚酸剂的选择……………………………………………………………15
3.3.5 pH值的优化……………………………………………………………15
3.3.6 提纯与精制………………………………………………………………15
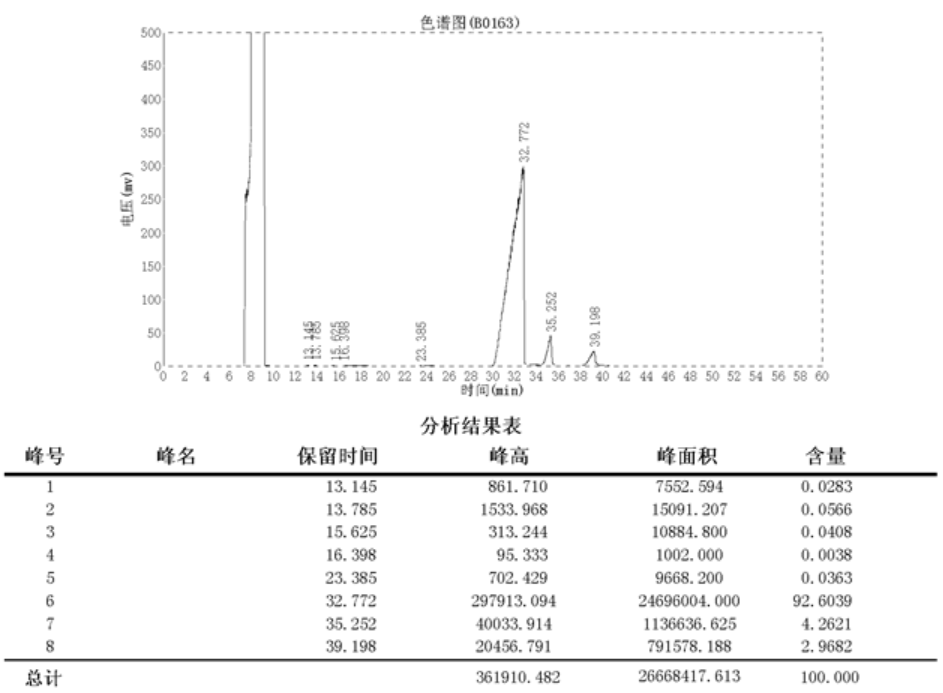
3.3.7 结果与讨论………………………………………………………………16
3.4 结构鉴定………………………………………………………………………...17
第四章 四氢环戊二烯醇衍生物的制备…………………………………….19
4.1 反应原理………………………………………………………………………...19
4.2 试剂与仪器……………………………………………………………………...19
4.2.1 试剂………………………………………………………………………19
4.2.2 仪器………………………………………………………………………19
4.3 实验部分………………………………………………………………………...20
4.3.1 使用叠氮化钠的注意事项………………………………………………20
4.3.2 实验步骤…………………………………………………………………20
4.3.3 实验条件的优化…………………………………………………………20
第五章 结论与展望……………………………………………………………..22
5.1 结论……………………………………………………………………………...22
5.2 展望……………………………………………………………………………...22
参考文献…………………………………………………………………………...23
致谢………………………………………………………………………………….26
第一章 文献综述
1.1前言
相关图片展示: