细菌漆酶在大肠杆菌中的表达及优化毕业论文
2020-05-20 21:11:02
摘 要
漆酶(laccase)作为一种重要的以铜离子为必须金属离子结构的多酚氧化酶,是一种典型的绿色催化剂,工业农业应用涉及领域广泛,在生物法环境修复、利用生物能源、食品工业检测和纸浆印染等废水处理等工业中有重要的研究价值和应用价值。其主要来源是真菌漆酶,但其在高温、高浓度盐存在或强碱性等工业极端环境中,真菌来源的漆酶因其抗逆性较低易失活,限制了其工业上的应用范围。而细菌来源的漆酶因功能上的差异,其相比较真菌漆酶会拥有更高的抗逆性和耐受性使其成为近年来学者们的研究热点。
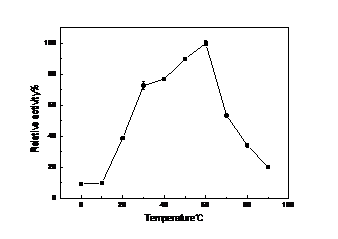
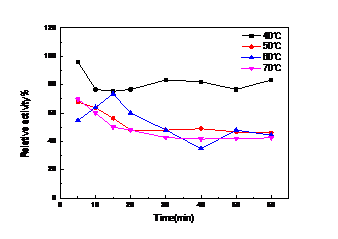
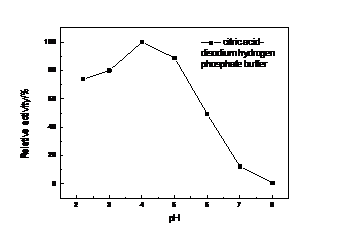
以得到生产酶的周期相对真菌漆酶较短,酶活高的细菌漆酶为目的,本文克隆的漆酶基因CotA来自枯草芽孢杆菌168(Bacillus subtilis 168),并在大肠杆菌BL21中进行原核表达。本论文采取的策略为从基因查询网站Genebank查询得知细菌菌株枯草芽孢杆菌168(Bacillus subtilis 168)菌株中的漆酶基因CotA序列全长为1542 bp,此基因编码513个氨基酸,加一个终止密码子。设计基因扩增引物后从提取的枯草芽孢基因组中克隆漆酶Cot A基因并构建表达质粒。将该质粒转化大肠杆菌E.coli BL21(DE3)菌株中通过利用IPTG诱导发酵法进行异源表达,最终获得的异源表达重组酶蛋白Cot A,并通过实验测得其酶学性质为最适的反应温度为60℃,最适的pH为4。且该酶在60℃还具有较好的热稳定性,在保温60 min后测得仍有65%的剩余酶活。可预见此酶在工业上有较好的应用前景。
关键词:漆酶;克隆表达;枯草芽孢杆菌;酶学性质
Gene cloning, expression of laccase CotA from Bacillus subtilis 168
Abstract
Laccase as an important polyphenol oxidase which use copper ions as an essential metal ion structure and the application range of industrial agriculture is very widespread in the biological environment restoration.It also has been used in detecting food industry waste and pulp dyeing wastewater treatment. So far, the main source of industrialized laccase is fungus fermentation, but the high temperature, high salt concentration or the presence of strongly basic industries in extreme environments.Fungal origin due to low resistance easily deactivated, limiting their industrial applications range. In contrast, bacterial laccase has short growth circle and can maintain the catalytic activity of enzyme under extreme environments.
For the production of the bacterial laccase cycle is relatively short, high bacterial laccase activity for the purpose of this paper, the bacterial laccase genes from Bacillus subtilis 168 were cloned and it’s sequences were heterologously expressed in host cell E. coli BL21 in this study . This paper use the method is the laccase genes were amplified from Bacillus subtilis 168 genome and the overall length of gene sequence of this gene was 1542 bp and it encoding 514 amino acid residues. Its predicted molecular weight was 64 kDa. The laccase gene of B. subtilis 168 CotA and expression vector pET20b were connected to construct recombine expression plasmids which were transferred into expression host cell E. coli BL21. The recombinant cell was incubated at lower temperature to keep micro-aerobic environment,which would show the activity of laccase.This laccase has the optimum temperature is 60 ℃, the optimal pH is 4. And the enzyme at 60 ℃ still has good thermal stability, there are still 65% of the measured residual activity at 60 min after incubation. This enzyme can be foreseen in the industry has a good application prospects.
Keywords:bacterial laccase;Bacillus subtilis 168 ;gene clone;heterologously expressed;
目 录
摘要 …………………………………………………………………………………………I
ABSTRACT ………………………………………………………………………………II
第一章 前言 ………………………………………………………………………………1
第二章 文献综述…………………………………………………………………………2
2.1 漆酶简介 ……………………………………………………………………………2
2.1.1 漆酶的生物学背景 ……………………………………………………………2
2.1.2 漆酶的应用 ……………………………………………………………………3
2.2 CotA芽孢蛋白研究概况 ……………………………………………………………4
2.3 课题来源及研究内容 ………………………………………………………………5
2.3.1 课题来源………………………………………………………………………5
2.3.2 本课题研究内容………………………………………………………………5
- 材料与方法 ……………………………………………………………………6
3.1 菌株与载体 …………………………………………………………………………6
3.2 主要实验试剂 ………………………………………………………………………6
3.3 主要实验仪器 ……………………………………………………………………7
3.4培养基配置及诱导物的添加 ………………………………………………………7
3.5 实验方法 …………………………………………………………………………7
3.5.1从芽孢杆菌漆酶基因组中克隆表达漆酶基因 ………………………………7
3.5.2 重组表达质粒pET20b/CotA的构建及鉴定 ………………………………10
3.5.3 重组菌的诱导表达 …………………………………………………………12
3.5.4 粗蛋白的制备…………………………………………………………………12
3.5.5蛋白质浓度的确定和聚丙烯酰胺凝胶电泳检测蛋白 ………………………13
3.6 漆酶活力的测定……………………………………………………………………13
3.7 重组枯草芽孢杆菌漆酶的酶学性质测试…………………………………………14
- 结果与讨论 ……………………………………………………………………15
4.1 芽孢杆菌属漆酶基因克隆及质粒构建 …………………………………………15
4.1.1 芽孢杆菌属漆酶基因克隆 …………………………………………………15
4.1.2 pET20b/CotA的构建及鉴定 …………………………………………………15
4.1.3 小结……………………………………………………………………………16
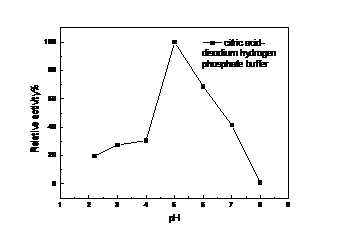
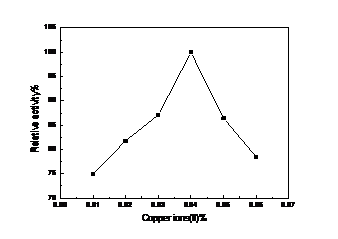
4.2 漆酶的酶活测定及酶学性质的研究 ………………………………………………16
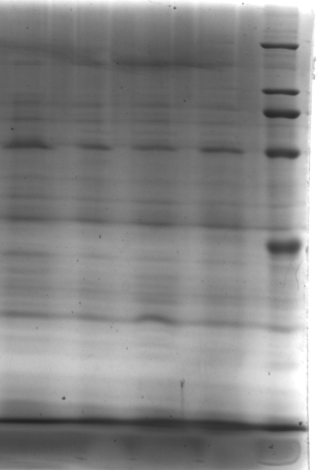
4.2.1 漆酶的获得……………………………………………………………………16
4.2.2 漆酶的酶学性质的研究 ……………………………………………………16
4.2.3 小结 …………………………………………………………………………21
第五章 总结 ……………………………………………………………………………22
参考文献 …………………………………………………………………………………24
致谢 ………………………………………………………………………………………25
相关图片展示: